Using organoids to evaluate the effect of toxins on S. Suis translocation

Fusarium mycotoxins such as deoxynivalenol (DON) frequently contaminate pig diets, often co-occurring with others like T-2 toxin. These toxins weaken intestinal barrier, raising infection risks. This study explores their effects on Streptococcus suis translocation using organoid models.
Fusarium mycotoxins such as deoxynivalenol (DON) commonly contaminate most pig diets (88%), often co-contaminating with other mycotoxins. For example, DON and T-2 toxin co-contamination is expected in 45% of globally produced diets. It is known that these mycotoxins compromise the intestinal barrier function, especially in young animals. Based on our observations, chronic exposure to low levels of these mycotoxins increases the susceptibility of piglets to pathogenic bacterial infections, requiring longer antibiotic therapy than piglets fed marginally contaminated diets. To understand this effect without conducting animal trials, we used a model that could mimic the pigs intestine to evaluate the translocation of Streptococcus suis during the exposure of porcine intestinal organoids to DON and T-2.
Set up of the study
Porcine ileal organoids were exposed to DON and T-2 individually or in combination and co-cultured with or without S. suis. To mimic chronic exposures as on-farm practice, DON was tested at a concentration of 0.1 µM and T-2 at a concentration of 0.01 µM, which corresponds to the expected concentration in blood after chronic dietary exposure to DON and T-2 at practical levels (e.g., 1 mg/kg DON and 0.1 mg/kg T-2). After 18-hours of in vitro culture, the integrity of the intestinal organoid monolayer was analyzed with a fluorescent dextran to detect the passage of non-electrolyte molecules, the transelectrical epithelial resistance (TER) to determine ionic barrier, as well as the translocation of S. suis (1 × 106 CFU/mL) across the intestinal layer were evaluated.
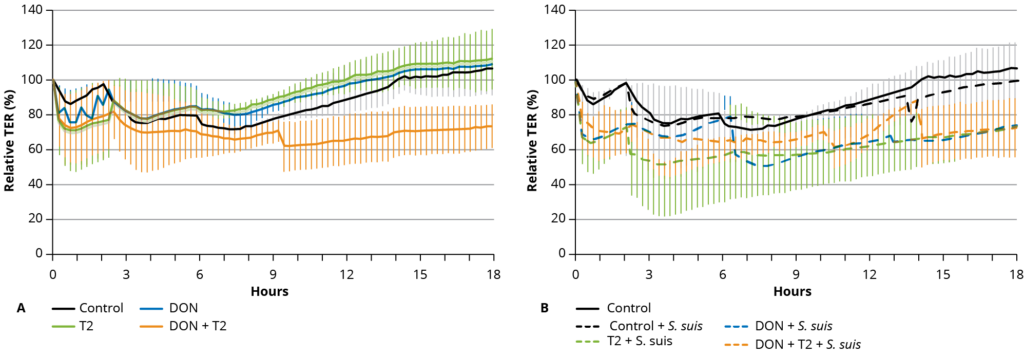
Figure 1 – Mean (± SD) transepithelial electric resistance (TER) of a 2D organoid culture.

Combination of mycotoxin and S. suis exposure
When testing intestinal leakage using fluorescent dextran (FITC-d), it was observed that exposure to mycotoxins alone did not significantly increase the permeability of the ileum organoid monolayers to solutes with a molecular size of 4 kDa. However, exposure to 1 × 106 CFU/mL S. suis in the control treatment led to an increase in permeability, which was further increased in combination with 0.01 µM T-2.
Changes in TER due to the different treatments were expressed as a percentage of the initial value at time zero (100%). At the start of the experiment, the TER values of the controls initially dropped to around 80% of the starting value and then gradually increased to 100% of the initial value over 18 h. A similar profile was observed when only 0.1 µM DON or 0.01 µM T-2 toxin was added to the apical culture medium (Figure 1A).
However, the combination of 0.1 µM DON with 0.01 µM T-2 toxin resulted in lower TER values over 18 h (Figure 1A). The addition of S. suis alone did not significantly decrease the TER over time, but in combination with 0.1 µM DON and 0.01 µM T-2 toxin, or the combination of mycotoxins, the TER value remained lower than 60% of the starting value between 6 and 18 h (Figure 1B) (p ≤ 0.05).
The decrease in TER values reflects an increase in the paracellular ion permeability of the monolayer, but this does not necessarily equate to the increased permeability of small molecules, indicating that it is possible to modulate one of these parameters without altering the other, and indicates here increased ionic conductance.
Figure 2 – Mean (± SD) S. suis colony forming units/mL (CFU/mL) in the apical (A) and basal (B) compartments of the culture wells.
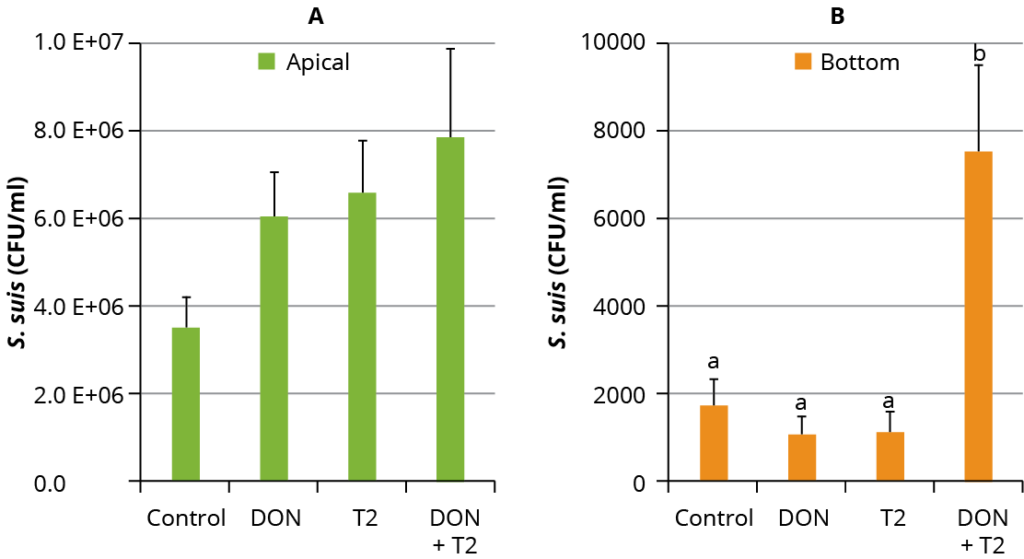
Quantification of S. suis translocation
To understand these findings, the translocation of S. suis was quantified by counting the bacterial colonies in the apical and basal compartments of the cultured material, where the presence of bacteria in the basal compartment indicated the bacterial translocation. The addition of mycotoxins did not affect the growth of S. suis in the apical part of the wells compared to S. suis alone (control) (Figure 2A). The addition of 0.1 µM DON and 0.01 µM T-2 together significantly increased the CFU of S. suis in the basal compartments compared to the controls and individual mycotoxin treatments (p < 0.0001) (Figure 2B).
Toxin exposure increases translocation of S. suis
It was concluded that concentrations of 0.1 µM DON and 0.01 µM T-2 toxin together can reduce the TER of the ileal organoid monolayer. The tested concentrations of mycotoxin are comparable to those measured in the plasma of pigs with a moderate dietary exposure. In combination with the mycotoxins, the translocation of S. suis across the epithelium was increased. The current model offers possibilities for testing the effect of dietary interventions on the porcine intestinal barrier function without conducting in vivo studies.











