A closer look at chelation
Nutritional science rarely strays into the realms of physical chemistry. When we look at effective mineral supply, however, a good knowledge of the physical interaction between transition metals and their ligands can help us to design the perfect supply for fine-tuning animal productions and health.
Much has been made of the positive responses seen when minerals are supplied as chelates, both in terms of health and productivity. While the inorganic forms of many transition metals have been considered adequate in the past for supplying essential minerals and trace elements, the extents to which animal production must become increasingly efficient in terms of productivity and waste disposal are making us reconsider not only the requirements for individual nutrients but also the form in which they are supplied. Because of this, it is vital that we understand fully the interaction between physiology and chemistry, so that we can be more precise in the way we satisfy specific demands. Modern livestock, racing and companion animals are more prolific, faster growing, more productive (meat, milk eggs), more active, more attractive (shiny coat) and must at least appear healthier than ever before. Conversely, they are exposed to more adverse and potentially unhealthy environments and are often much more sensitive to these as they struggle to satisfy their genetic potential.
Bioavailability is crucial
While it is widely accepted that the secret of supplying minerals is to improve the amounts that are available for metabolic purposes-the bioavailability, the way in which this is done does not always reflect our understanding of animal physiology. While there are now many products available on the market that supply trace elements in an organically bound form, in the past there was no way of identifying the strength of the binding; how much of the metal was actually bound at any given pH; or, most importantly, how the chelation strength affects the bioavailability of the metal. It is not only important that the transition metal remains chelated until it is incorporated into metabolically active compounds, when it should be released (thus chelation strength should be neither too weak nor too strong), but metal ion and ligand concentrations should preferably be maintained optimally at a variety of feed, intestinal and intracellular pH values.
The electrochemistry of chelates
The strength of metal-ligand bonds varies with the metallic element, the positive charge of the metal cation, the ligand atom which bonds to the metal, e.g. oxygen, nitrogen or sulphur, and the number of points of attachment utilized by chelating agents. When it comes to the interactions between metals and their ligands, the differences between relative bonding strengths in complexes or chelates can be quite large.
Work conducted by Robert Holwerda’s team at Texas Tech University in the mid 1990’s set the scene for the development of effective solutions to the problem of supply and demand for trace elements. They developed an electrochemical test that can be used to identify the relative amounts of free and chelated metal ions in aqueous solutions of organic minerals in vitro or in vivo. The method is based on an electroanalytical technique known as polarography. In a simplified version of this method, an increasingly negative potential is applied to a hanging mercury drop electrode in the absence or presence of a dissolved chelating ligand until a current is drawn from the reduction of transition metal ions into the amalgamated metal (e.g. from Zn2+ to Zn(Hg), where all chelation is lost). In brief, electrochemical data can be translated into a formation constant, Kf, which gives an indication of the strength of the metal-ligand bond and the average number of ligands bonded per metal ion, p. Knowing the value of p is vital in distinguishing complexing from chelating ligands. Given that zinc can typically form four bonds, a polarographic p value of 2 would indicate chelation with a pair of bidentate (two different bonds per ligand) ligands, while a p value of 4 would indicate a simple complex formed by four non-chelating ligands. For routine evaluation of chelation effectiveness, a formation quotient, Qf, is calculated from the negative shift in reduction potential linked to metal-ligand bonding. Qf values provide a practical measure of metal-ligand complexing strength that is particularly helpful when comparing different proteinate products. The scale ranges from 1 (no chelation, only free metal ions present) to the extremely powerful chelating ability of EDTA, with a Qf value on the order of 1012.
Not all chelates are the same
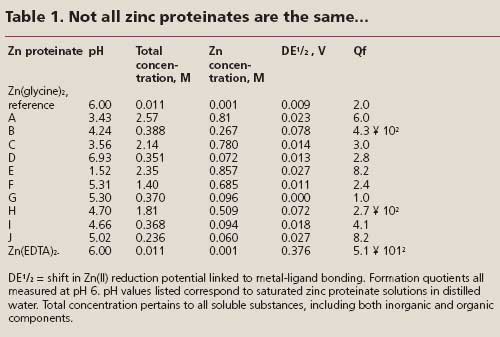
Table 1 shows an electrochemical evaluation of a number of commercially available zinc proteinates, using Zn(glycine)2 as a reference chelate. It can be seen clearly that solubilities vary greatly between products. Most zinc proteinates are internally buffered so that their pH falls in the weakly acidic range of 4-6. On the basis of their Qf values, Zn proteinates A, C, D, E, F, I and J are relatively weakly chelated in solution, similar to a typical 1:2 Zn-amino acid complex. Proteinate G’s chelation properties are so weak as to be indistinguishable from uncomplexed zinc sulphate. Zinc proteinates B and H, however, do contain strongly chelated zinc.
The standard Qf assay is conducted at a constant pH of 6.0. However, it is also useful to know how the quality of chelation changes with pH. This will give an idea of proteinate suitabilities for feed applications. Figure 1 shows a pH variation study of Zn proteinates A, B and C. The percentage chelated zinc at each pH was calculated from Qf, ligand acid ionisation constant Ka and total ligand concentration values. Chelation efficiency increases in all cases with increasing pH. However, there are clear differences between the three different proteinates tested with respect to their stability. While proteinates A and C are both largely unchelated below pH 5, 60% of proteinate B remains chelated all the way down to pH 4. Furthermore, the ranking B>A>C based on the Qf value at pH 6.0 holds true for all other pH values.
In practice…
Physiologically, the metal ion-ligand equilibrium is extremely important. From the pH of the feed formulation, to the wet bolus, through digestion, absorption and to metabolism in the cells of visceral organs muscle, connective tissue, enzymes etc., the metal has to be in the correct form to either protect it or release it, depending on the ambient physiological conditions. Evolution has equipped animals to utilise minerals and trace elements from a variety of chemical sources and thus in many forms. However, the efficiency of their use, especially when it comes to the energy involved in transport across cell membranes and incorporation into metabolically active compounds depends upon the chemical form in which they reach their target organs, cells and organelles. Holwerda’s methodology shows quite clearly that not all chelates are the same, especially when it comes to their stability at physiological pH. This inevitably has knock on effects in terms of the ability of different chelates to satisfy metabolic demand. Knowing the chemistry is therefore vital if we are to be able to supply the metal elements in the diet as effectively as possible.
STRESSED BROILERS REAP THE BENEFITS OF CHELATES
As with many nutrients that are required only in trace amounts, a small improvement in the dietary supply of trace metals can often be used to best effect in times of metabolic stress. Improving mineral supplementation is one of the strategies adopted in the control of malabsorption syndrome- a complex enteric aetiology affecting young chicks thought to be viral and in origin and reports thus suggest that it is infectious. Among the clinical signs of malabsorption syndrome, stunted growth, high early mortality, weak legs and the presence of undigested feed in the faeces suggest a general decrease in digestive function, pale skin (indicating anaemia) abnormal feathering and central nervous system malfunction (tremors and incoordination) are all more specifically indicative of mineral and trace element deficiency, most probably as a direct result of the decreased digestive capacity. More common in broiler houses is the problem of heat stress. Rather than affecting nutrient uptake and utilisation directly, heat stressed birds can indirectly benefit from improved mineral and trace metal supply because of their often dramatically reduced feed intake at high temperatures.
To demonstrate that improving the form in which dietary zinc and manganese are supplied can have positive benefits during periods of metabolic stress for broilers, a trial was conducted at Nutreco’s poultry research centre in Spain. A combined stress (malabsorption syndrome infection and high temperature, Table 1) was imposed on one group of 624 female broilers. Groups of stressed and non-stressed birds were offered diets in which the zinc and/or manganese was supplemented either entirely in an inorganic form, or 30% of the supplement in the premix replaced by either chelated zinc (Optimin Zn), chelated manganese or both (Optimin Mn) or both (Optimin Zn-Mn, Trouw Nutrition, USA).Although no significant effects of including chelated zinc or manganese in the diets of non-stressed (control) broilers were observed, there were some tendencies towards higher body weight and lower FCR when both Zn and Mn were partially replaced with chelated forms. These birds did show significantly improved final body weight and average daily weight gain.
The most significant effect of the chelated minerals was that on mortality in the birds subjected to infection and heat stress. There appeared to be a positive effect on mortality by the use of chelated zinc, chelated manganese or both (Table 2), although the negative effects of stress on weight gain, body weight and feed intake could not be reversed.
HIGH LEVELS OF CHELATES IMPROVE DAIRY RESPONSES
Milk production and reproduction in dairy cows exerts a high demand on all nutrients. A study at Mississippi state university in the US was thus designed to investigate whether trace elements could be better provided in chelated form.
Forty eight Holstein cows were separated into three groups of 16 and given a diet based on of hay, haylage and maize silage balanced with a concentrate and supplemented with different sources (inorganic or inorganic plus chelated) and levels of the trace minerals zinc, copper, manganese and cobalt as shown in Table 1. Diets were fed from 21 days pre-calving and for the first 150 days of lactation. Milk weight (cows were milked twice daily) was recorded daily and milk samples analysed for fat, protein, lactose and the somatic cell count (SCC) measured. The reproduction data collected include days to first oestrus; days to first service; services per conception and days calving to conception. Samples of liver and uterine endometrium were taken during the study (uterine endometrial samples taken at calving only) and analysed for content of zic, copper and manganese.
Cows supplemented with the chelated minerals produced more fat-corrected milk, had lower SCC scores, fewer days to first oestrus, days to first service, days between calving and conception and fewer services per conception than either the control animals or those supplemented with inorganic minerals alone (Table 2). No differences were observed between the treatments in tissue status for zinc, copper or manganese. Economically, the milk and reproductive data together resulted in an increase in profit of at least $110 per cow as a result of the use of chelated mineral supplements and a return on investment of more than 16:1.
PROTEINATED TRACE MINERALS IMPROVE WEANER PERFORMANCE
Weanling pigs are at a particularly delicate stage in their development and are especially prone to nutritional stress. Thus, a trial was conducted to investigate the effects of supplementing part of the trace element requirement in a proteinated form on growth and feed efficiency parameters.
Ninety six pigs weaned at 21 days of age, weighing an average of 6.34 kg were assigned one of two diets. The control diet was supplemented with iron, zinc, manganese, copper, selenium and iodine as sulphates. The iron, zinc, manganese and copper content of the test group was partially made up in the form of proteinates, as illustrated in Table 1. The feeding trial was split into two phases: phase I (days 0-14 post-weaning) and Phase II (days 15-28).
The results of the feeding trial are summarised in Table 2. Pigs fed the diet containing mineral proteinates were significantly heavier on days 14, 21 and 28 after weaning. During Phase I, the pigs supplemented with the mineral proteinates had significantly (P<0.04) greater average daily gain and improved FCR. The same pigs also showed significantly better growth results over the whole trial period.
HEALTHY PUPS AND GLOSSY COATS
The reproductive potential of dogs is an area not often studied in relation to nutrition, indeed, at first glance it is of interest only to breeders. However, as with livestock, giving pups the best start possible has knock on effects in terms of their later nutrition as working or companion animals. As with all breeding animals, mineral nutrition is coming under increasing scrutiny with respect to health and productivity. Dietary deficiency in trace minerals is commonly manifested in breeding bitches as an inability to establish or maintain a pregnancy or a reduction in the size of the litter. Collaboration between American petfood manufacturer Pet Products Plus Inc. and DuCoa Ltd. In 1998 resulted into a rare insight into the value of supplementing the diet of breeding bitches with chelated, rather than inorganic trace elements (Kuhlman and Rompala, 1998). A total of 34 individual beagle bitches were randomly assigned to one of two diets- a control or a diet in which chelated forms of zinc, manganese and copper replaced part of the inorganic supplement. Dogs were fed their respective diet for at least two weeks before oestrus, through gestation and during lactation. All bitches were given a physical examination at the beginning of the study, weekly during gestation, at whelping and then weekly until week six of lactation, when pups are commonly removed. The litter size at birth and at 1 and 4 days of age were recorded as well as stillbirths and congenital abnormalities. Puppies were given a physical examination at whelping at four weeks of age.
No significant differences in the food intake or body weight of the bitches were recorded during the study. Pooled whelping data (Table 1) showed that bitches consuming the diet supplemented with chelated Zn, Cu and Mn had more (P<0.05) pups than those fed the control diet. No differences were observed in the growth rate of the pups between treatments and all pups showed a steady weight gain from birth until weaning at 6 weeks of age. The improvement in litter size is comparable to that seen in sows and has been associated with greater early embryonic survival and lower rates of embryonic death.
When hair samples were taken from the bitches, although the mineral content of the hair was not significantly altered by dietary treatment, scanning electron microscopy revealed that hair follicle condition was improved in the bitches supplemented with the chelated minerals (Figure 1). Hair taken from the group supplemented with the three trace elements in chelated form appeared smoother and less fragmented. Zinc is known to be an essential component of hair growth and other epidermal tissue. It is believed that, as with pigs and many other animals, chelated trace elements are more readily absorbed and retained longer than their inorganic counterparts
Reference
Kuhlman and Rompala (1998). J. Nutr. 128 Supplement 12S pp. 2603-2605S.











