Balanced trace element nutrition to neutralise oxidative stress
High iron levels in feedstuffs and water used in animal husbandry are common around the globe. Excessive amounts of iron are fed when comparing animal requirements with intake from feedstuffs. Although it is an essential mineral, feeding iron in excessive amounts can cause oxidative damage to tissues. On the other hand, feedstuffs do not supply sufficient amounts of zinc, copper and manganese. Balanced and bioavailable trace element supply will protect the animal from oxidative stress.
By Paul Perucchietti and Wilbert Litjens, Trouw Nutrition Feed Additives, Tilburg, the Netherlands
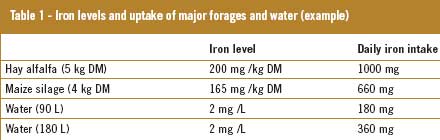
The levels of iron in the soil correlate with the levels of iron in groundwater. High levels of this mineral are observed in a large part of the world. The maximum level of iron allowed in drinking water for human consumption is 0.2 mg/l (EU directive). When the level exceeds 0.3 mg/l, the colour, smell and taste of the water will be affected. The level of this mineral in groundwater can rise up to 100 mg/l, resulting in an excessive oversupply of iron from the water, on top of the iron from feedstuffs. The levels of iron in feedstuffs grown on soil high in iron are usually higher than average estimates. Iron toxicity is a particular risk on dairy farms where cattle depend on local groundwater and forages. Also, it is important to note that during the summer months, and at peak lactation, the animals will increase the amount of water they consume, further increasing iron uptake. When all these effects are combined, the level of dietary iron can be at such a level that the performance of the animal decreases (i.e. retained placenta, udder oedema). The iron requirement of an adult dairy cow is estimated to be about 100 mg/day, depending on dry matter intake and production. The estimated uptake and excessive supply are listed in Table 1.
Iron as pro-oxidant
Iron can also act as a pro-oxidant. The following Fenton-type reactions result in the production of reactive molecules:
O2− + Fe3+ → O2 + Fe2+
H2O2 + Fe2+ → Fe3+ + OH− + OH
Many types of macromolecules, like lipids, nucleic acids, sugars, amino acids and proteins, are affected by this oxidative stress. Under normal circumstances, the harmful effects of iron are inhibited by complexing the mineral in larger molecules that are transported from sites susceptible to damage. Dietary imbalances, inflammation, environmental stresses or saturation of iron-binding sites are likely to negatively influence this process. Elevated concentrations of iron in tissues in humans correlate with increased protein carbonyl concentrations, which indicate oxidative damage. High tissue iron concentrations are associated with the development and progression of several conditions, like certain cancers, liver and heart disease, diabetes, hormonal abnormalities and immune dysfunctions.
Antioxidative properties
A deficiency in zinc, manganese, copper or selenium can contribute to tissue oxidative damage. These are the active components of well-known antioxidant enzymes such as superoxide dismutase (SOD) and Glutathione peroxidase (GSH-Px). Moreover, zinc is also associated with metallothionein (MT), which can act as an antioxidant. Furthermore, zinc competes with iron for membrane-binding sites. Zinc has a higher affinity for these binding sites, so at equal concentrations zinc is more likely to bind than iron. Binding of zinc instead of iron reduces the risk of site-specific damage caused by the formation of hydroxyl radicals (OH). Additionally, zinc has a stabilising effect on organelles and macromolecules.
Research has shown that zinc can act as specific antioxidant protecting macromolecules against the negative effects of iron-induced oxidative stress. It was shown in periparturient dairy cows that zinc is an effective antioxidant when iron is fed in excessive amounts (12 g per cow as FeSO4∙7H2O). Vitamin E was only effective as an antioxidant at low iron dietary levels, but ineffective at high levels. Further research in humans provided similar results when daily dietary zinc was supplemented. Plasma levels of lipid peroxidation products and DNA adducts decreased. Figure 1 shows the metabolism of pro-oxidants and antioxidants.
Iron and zinc absorption
Metals can be actively and passively absorbed through the cell wall of the small intestine. Passive absorption occurs when excessive amounts are fed; this cannot be controlled by the animal and is neither predictable or efficient. Active absorption is a controlled process whereby the animal will absorb the amount of minerals necessary to fulfil its needs. Zinc, copper, manganese and iron are all cations and compete for the same cation absorption and transport proteins. When iron is present at relatively high levels, it will lead to depressed absorption of other trace minerals. Chelated minerals prevent this negative effect by allowing the animal to actively absorb the required mineral. As there are many organic minerals in the market, it is not always easy to identify a proper chelated mineral that will help to overcome these problems. First of all, the chelate must remain soluble, as this is essential for absorption. Secondly, the mineral should not react with other feed components. By increasing the internal bond strength of a chelate, both can be achieved. Trouw Nutrition markets Optimin minerals with the highest bond strength available, ensuring an optimal and balanced mineral supply.
Conclusion
High iron levels in feedstuffs and water cause an overload of dietary iron. This may lead to lowered absorption of zinc, manganese and copper. An excess of iron leads to oxidative stress, which is harmful to the animal and inhibits optimal animal performance. Zinc and other trace elements have antioxidative functions and may help the animal to overcome the effects of high iron levels. Frequent analysis of water and forages will bring more accuracy in formulating the most efficient level of dairy cow trace mineral supplementation. A partial replacement of inorganic minerals (Optimin) by organic minerals will prevent a deficiency of trace minerals, and oxidative stress in animals exposed to high dietary iron levels.
References and more info can be obtained from the author at Wilbert.Litjens@nutreco.com. For further questions Trouw Nutrition is also available at EuroTier exhibition in November in Hannover, Germany.











