FDA considering approval GM salmon
The US Food and Drug Administration (FDA) is considering whether to approve the first genetically engineered salmon for human consumption. The fish can grow at twice the normal rate.
AquaBounty Technologies, who developed the salmon, has been trying to get approval for a decade.
Now the company seems to have submitted most or all of the data the FDA needs for analyzing whether the salmon are safe to eat, nutritionally equivalent to other salmon and safe for the environment, according to government and biotechnology industry officials.
A public meeting to discuss the salmon may be held in autumn of this year.
Mixed opinions
Some consumer and environmental groups are likely to raise objections to approval. Even within the FDA, there has been a debate about whether the salmon should be labelled as genetically engineered. In contrast; genetically engineered crops are not labelled.
Approval of the salmon would help open a path for companies and scientists developing other genetically engineered animals, such as cattle resistant to mad-cow disease or pigs that could supply healthier bacon.
After the salmon, next in line for possible approval would probably be the "enviropig," developed at a Canadian university, which has less phosphorus pollution in its manure.
Growth hormone
AquaBounty Technologiessaid the salmon would be raised in fish farms. It is an Atlantic salmon that contains a growth-hormone gene from a chinook salmon and a genetic on-switch from the ocean pout, a distant relative of the salmon.
Normally, salmon do not make growth hormone in cold weather. But the pout’s on-switch keeps production of the hormone going year round.
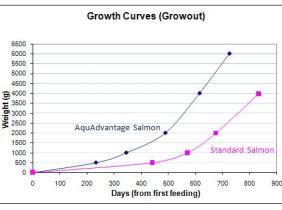
The result is salmon that can grow to market size in 16 to 18 months instead of three years (see graph), though the company says the modified salmon will not end up any bigger than a conventional fish.
AquaBounty, based in Waltham, Massachusettes, said last week that the FDA had signed off on five of the seven sets of data required to demonstrate that the fish is safe for consumption and for the environment.
The company said that if approved it would take another two or three years for the salmon to reach supermarkets.











