Managing the gut microbiota to optimise laying hen productivity

A dramatic increase in antibiotic resistance worldwide has led to a ban in their use as growth promoters in many countries. This situation led to a higher risk of an overgrowth of pathogenic microflora in the intestine, associated with enteric disorders, mortality, and diarrhoea signs, leading to a strong reduction of growth and feed intake.
Antibiotics are administrated for therapeutic reasons at a curative dose, or they can be administrated at a preventive dose to avoid unwanted bacteria growth in the intestine and stimulate growth performances. Currently, there is controversy surrounding the use of antibiotics and, in particular, antibiotics used as growth promoters (AGP) for animals destined for meat and eggs production.
Antimicrobial resistance appears when the micro-organisms which cause infection change over time and develop the ability to survive to the exposure of a drug that would normally kill them or stop their growth. This phenomenon allows those strains to grow and spread due to a lack of competition from other strains. This has led to the emergence of ‘superbugs’, or bacteria which are difficult or impossible to treat with existing medicines. Moreover, foodstuffs (e.g., meat, eggs) can be a vector of transmission of those resistant bacteria.
According to the Review of Antimicrobial Resistance (2016), without policies to stop the spread of antimicrobial resistance, the increasing number of deaths every year would become an extremely disturbing 10 million every year, which is more people than currently die from cancer. The review also took as an example another consequence of antimicrobial resistance in India where antibiotic-resistant neonatal infections cause the deaths of nearly 60,000 newborns each year.
Consequently, there is a need for alternative products able to control gut microbiota. Various antimicrobial solutions have been developed as feed additives including direct antimicrobial products like acidifiers or vegetal extracts or indirect antimicrobial products like probiotics or prebiotics. Those types of products are the most known, but not the only ones able to act as antimicrobials in animal nutrition.
CeC mode of action
To support laying hens’ microbiota and growth during challenging periods as the start of lay and the production peak, but also to ensure a well-balanced and secure microflora during the whole life of the animals, a unique and patented Copper exchanged Clay (CeC) has been developed by Wisium. This solution is a combination of copper ions at a very low level (200 times lower than the copper content in poultry feed permitted in the EU) and a synthetic zeolite having anti-microbial properties and able to specifically target pathogenic bacteria and have limited action against beneficial bacteria.
The results of several in vitro trials are available and highlight the benefits of CeC over different strains of bacteria found in farms involving infections, clinical consequences as liquid faeces, mortality and leading to economic loss.
As an example, human non-typhoidal Salmonellosis is the second most frequently bacterial disease coming from food encountered in Europe, after Campylobacteriosis.
Salmonella spp. are mainly located in the poultry intestinal tract. Consequently, faeces can be contaminated with Salmonella spp. and became a hazardous source for consumers through the consumption of eggs and meat.
In Europe, 5 Salmonella serotypes are regulated for the poultry species (Gallus gallus) as they are considered to represent a health hazard: S. enteritidis, S. typhimurium, S. hadar, S. infantis and S. virchow.
Other Salmonella strains can also contaminate poultry as S. gallinarum which involves the fowl typhoid infection and can cause diseases mainly in adult or growing chickens and turkeys. Acute cases of infection are related to septicaemia. In subacute outbreaks, there are dead-in-shell embryos, or dead chicks on the hatching trays. Chronically affected birds show anaemia and focal necrosis in the liver, heart, intestines, and pancreas.
CeC is efficient on pathogenic bacteria independently if they are gram-negative (Escherichia coli, Salmonella spp.) or gram-positive (Clostridium perfringens, Streptococcus suis).
Figure 1 demonstrates the efficiency of CeC on Escherichia coli strain, Clostridium perfringens, Salmonella enteritidis, S. typhimurium, S. hadar, S. infantis and S. gallinarum. With CeC, bacteria population was decreased from 4 log10 to 8 log10. It is commonly considered that 4 log reduction corresponds to a reduction of 99.99% of the bacterial population and 5 log to 99.999%.
Figure 1 – Inhibition curves with the application of CeC
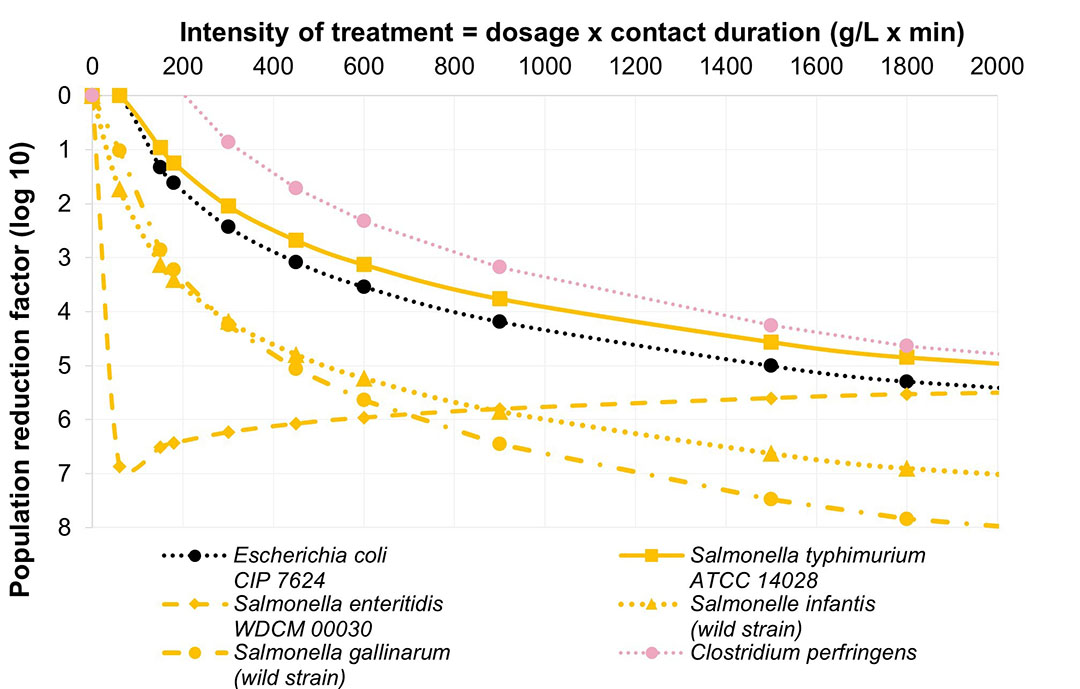
Consequently, CeC can potentially reduce the microbial population of all the strains tested, responsible for diseases on laying hens and could be considered a solution helping in the control of Salmonella pressure in the animal microbiota. This selective modulation of the gut microbiota thanks to CeC has also been proved through published in vivo trial.
Optimisation of productivity
The link between the necessity to have a well-balanced microbiota and productivity is not new but more recent scientific information highlights the strong role that microbiota can also play in another physiologic role.
CeC is backed by scientific evidence on laying hens based on trials run on field conditions and on R&D farms. Thanks to a better gut microbiota control, CeC improved the performance of layers in comparison with a negative control.
To illustrate this effect, a trial run in field conditions in Brazil showed that after 5 weeks of supplementation with CeC, on birds 46 weeks old, a reduction of 96% of Clostridium in the faeces was measured compared to the control group and confirms the results observed in vitro. The number of dirty eggs was reduced thanks to CeC and consequently, the number of eggs suitable for sale increased. Performances obtained in the CeC group were also higher than those obtained in the zinc bacitracin group, an antibiotic growth promoter.
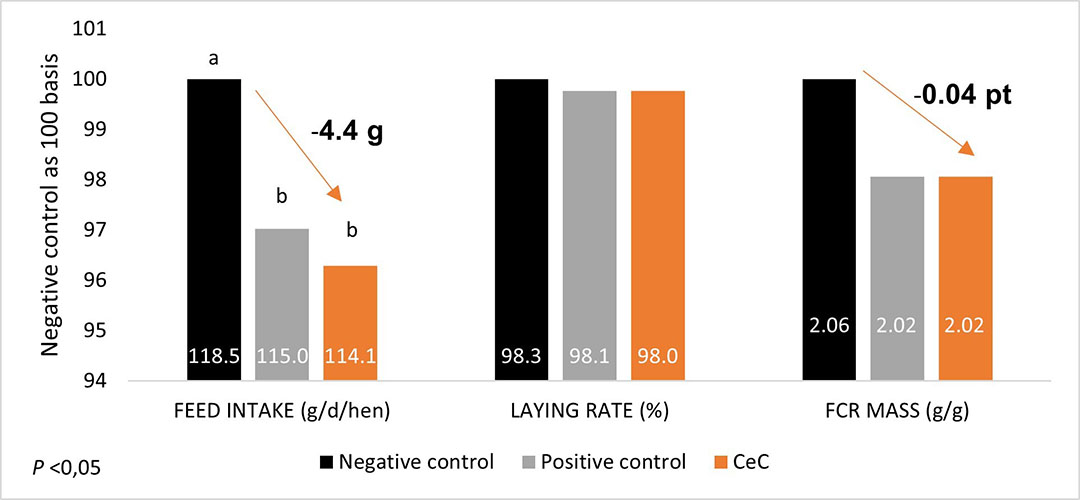
A more recent trial run in a Brazilian university (FZEA/USP) on Novogen brown layers supplemented with CeC from 18 to 38 weeks of age highlighted a significant reduction of the feed intake of -4.4 g/hen (P<0.05) in comparison with a control group while animals maintaining a similar laying rate and leading to a reduction of the FCR by -0.04 pt.
Additionally, this trial again demonstrates that CeC obtained equivalent results than an antibiotic growth promoter (halquinol) (Figure 2).
As a result, CeC could be considered an efficient solution for laying hens’ production, to improve performances thanks to a better effect on microbiota.
Figure 2 – Zootechnical performances of laying hens supplemented from 18 to 38 weeks of age with CeC or Halquinol (positive control)
CeC, an efficient solution
The in vitro inhibition trials on several pathogenic strains commonly found on laying hens farms and especially E. coli, Salmonella and Clostridium strains showed that CeC could inhibit both the gram-negative and gram-positive bacteria strains tested. Based on these in vitro trials, CeC could be considered an efficient solution to help in reducing the main pathological bacterial population in the gut.
Obviously, in vitro trials alone are not sufficient to validate the efficiency of a feed additive. That is why in vivo trial results confirmed in vitro observation: by helping to promote a well-balanced gut microbiota of laying hens, CeC involves a regular and reliable improvement of performances, specifically about FCR and participates in the reduction of discarded eggs.
*References are available on request


