Toxic and unstable? Choose your selenium source carefully

The type of selenium and the form in which it is supplemented plays a crucial role in its safety, bioavailability and efficacy. A well-considered choice of selenium source is essential.
Selenium supplements are available in several forms; inorganic mineral salts, such as sodium selenite or selenate; organic forms, such as selenium-enriched yeast in which selenoamino acid analogues such as selenomethionine predominate; or chemically synthesised selenoamino acids and selenoamino acid analogues produced synthetically. The distribution and accumulation of selenium and selenium form in animal tissues depends greatly on the type of selenium supplement, and the form in which selenium is presented plays a crucial role in its bioavailability and efficacy. Organic forms of selenium are the optimal nutritional source, and upon uptake within the cell, they get transformed into common seleno-intermediates for further use and/or excretion.
High SeMet content does not mean high bioavailablity
There is a misconception within the feed industry regarding the total SeMet content of selenium products with the belief that “more is better.” Such arguments have no scientific basis and there is, indeed, no evidence that increasing the level of SeMet will equate to a better product. While the level of SeMet may vary among products, it is also to be anticipated that the bioaccessibility and availability of the SeMet that is released by the digestive processes in the GI tract will also differ. Critically, what is becoming increasingly clear from a research perspective is that the form in which selenium is presented will influence the stability and thus the cellular reactivity of the molecule. Less stable forms of selenium have enhanced toxicological properties, while those of more stable preparations are far less toxic.
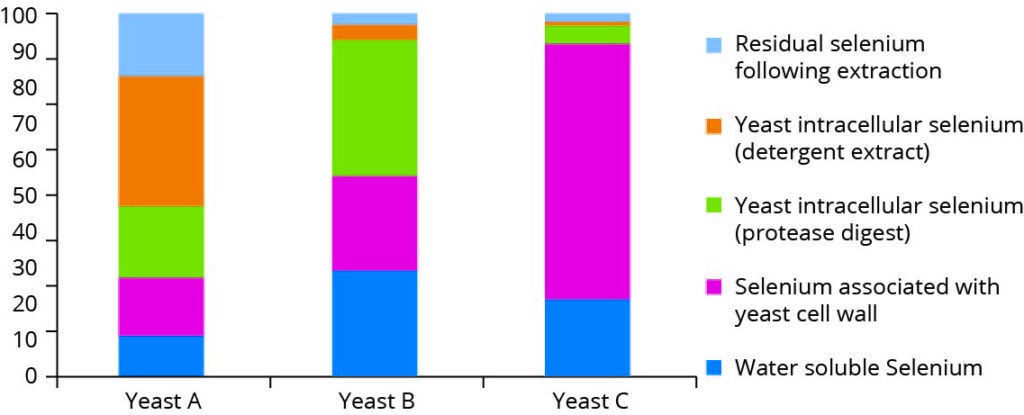
In one of the first studies, which demonstrated the uniqueness of individual selenium products, the authors examined three different commercial preparations of selenium-enriched yeast, subjecting each to a series of sequential extractions followed by various enzymic digestions designed to liberate selenocompounds which are associated with various polysaccharide and protein fractions. These compounds were subsequently separated and speciated by SEC-ICP MS, and the recoveries in the various fractions from each yeast product compared (Figure 1). The results outlined in Figure 1 monitor the fractionation of the selenocompounds in yeast by using different extraction techniques.
Figure 1 – Selenium associated yeast fractions (adapted from
Encinar et al, 2003)
All selenium yeasts are not the same
Although there is a very common perception that all selenium yeast preparations are the same, this is not the case, and it is clear that the compartmentalisation of selenium within yeast is totally different between preparations. Just as there are differences among yeast strains at a genetic level, there are fundamental differences in the way yeasts distribute selenium within the cell.
As subcellular deposition of selenium within selenium-enriched yeast preparations is so widely different, it is reasonable to expect that these preparations will also differ in parameters such as shelf life, bioavailability and, indeed, toxicology. Rather than viewing these products in the same light, they must be seen as distinct selenium preparations.
Given the highly regulated nature of the use of selenium and selenium products, one can easily find much information in relation to areas such as selenium source toxicity and stability. This type of information allows end-users to make informed decisions as to the suitability of individual products for both their production systems and for end use.
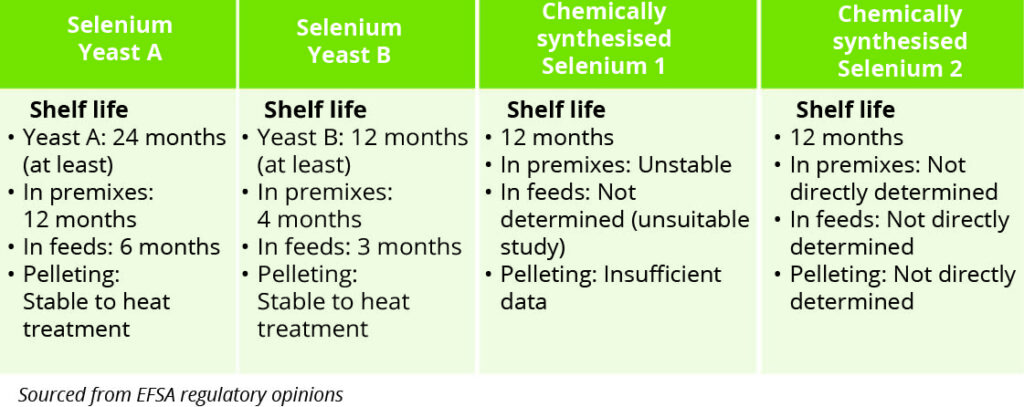
Table 1 – Selenium source stability
Selenium source stability in premix and feed
Taken from regulatory opinions on the materials, a differential examination of the available stability data on organic selenium yeast and the synthetic selenium sources is highlighted in Table 1, from which notable differences between them can be appreciated.
Perhaps some of the most striking comparators are the notable differences between their stabilities in the premix. The instability of one of the synthetic selenium sources was quite noticeable, with recovery after three, six and nine months being reported as 55%, 54% and 37%, respectively.
Examination of the other synthetic source did not show the measured stability of the actual compound and instead reported it for a non-selenised variant molecule. Additional questions remain about the stability of the synthetic sources in compound feed and after pelleting, with either insufficient data being generated or no direct measurement of stability being made.
In contrast, the organic selenium yeast sources display high levels of verified stability in premix, compound feed and after pelleting, albeit with further source-dependent differences noted between them.
Given the rising costs of raw materials and additives, premix and feed manufacturers are unsurprisingly placing increasing scrutiny on formulations and, in particular, on the stability of individual materials. Potential losses due to interactions in the premix or as a result of pelleting are of considerable concern.
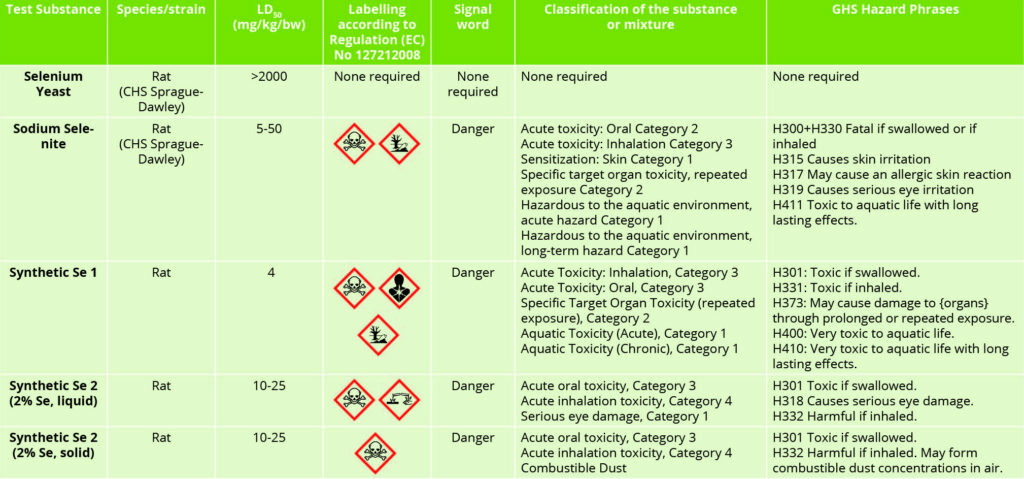
Table 2 – Selenium source toxicity and hazard labelling.
Click here for enlarged image of table
Differences in toxicity between selenium sources
Table 2 highlights the differential toxicities associated with inorganic, organic and chemically synthesised sources of selenium. The quite distinct and notable differences between selenium sources are of interest in this data. The organic selenium yeast product has relatively little, if any, negative connotations associated with its use.
In contrast, the inorganic and synthetic selenium sources have clearly defined toxic attributes, which are notably quite similar with respect to their acute oral toxicities. The subtleties associated with the toxicities of liquid and solid forms of the same synthetic selenium source are also of interest. Clearly, when considering selenium sources, one cannot class them as all being equal.
The reasons behind the toxicity differences
In terms of understanding the toxicity differences among selenium sources, it can be useful to examine the biochemistry behind selenium and its potential to act negatively at a cellular level.
The pro-oxidant properties of selenium sources, such as sodium selenite, originate from their conversion into selenide or selenols, which are readily oxidised and generate reactive oxygen species (ROS). The toxicity of selenite is well documented as being mainly caused by DNA damage due to the induction of ROS-dependent DNA strand breaks and/or base oxidation, leading to apoptotic and necrotic cell death.
More recent research has shown that freely accessible selenocompounds (such as chemically synthesised selenium) can have pro-oxidant properties, which are initiated in the same manner as sodium selenite but can be further enhanced due to the initiation of additional cycles of oxidation/reduction. Ultimately these redox cycles consume intracellular antioxidants such as GSH and, consequently, the reducing cofactor NADPH. Not only can such selenocompound-induced redox cycling lead to antioxidant imbalance; it can also lead to increased and sustained production of ROS, which can further damage nucleic acids, proteins and lipids.
In addition, oxidised selenols will catalyse the formation of disulfide bridges between low molecular-weight thiols and proteins, potentially leading to protein inactivation or aggregation.
Newer studies indicate that the toxicity of freely accessible selenocompounds results from their conversion into selenocysteine, a selenoamino acid with the ability to mediate proteotoxic stress, thus playing a role in selenium toxicity that was underestimated until now.
The inherent risk of toxicity from selenocysteine mediated proteotoxicity requires further research to fully understand the implications associated with its cellular generation. It may well be that the enhanced toxicities of the chemically synthesised analogues are due to increased redox cycling and/or increased induction of proteotoxicity due to enhanced selenocysteine synthesis at a cellular level, leading to acute oral toxicities of similar impact to that of inorganic sodium selenite.
Selenium yeast has much lower toxicity
A benefit that organic selenium yeast provides in this regard is the stabilising influence that peptide and protein incorporation of selenium provides. By incorporating selenium into peptides and proteins rather than amino acids, the potential for instability is vastly reduced.
Significantly, the impact on ROS-mediated DNA, protein and lipid damage at a cellular level is negated. In contrast, chemically synthesised sources do not have the protecting benefit of yeast protein incorporation and have been shown to have negative cellular impacts.
The instability of synthetic selenoamino acids is well documented. Older studies, for instance, highlighting this phenomenon, subjected samples of synthetic L-selenomethionine and organic selenium yeast to oxidising conditions in the form of a peroxide challenge.
Figure 2 – The effect of oxidizing conditions on the recovery of synthetic L-SeMet and peptide bound SeMet (Selenium yeast).
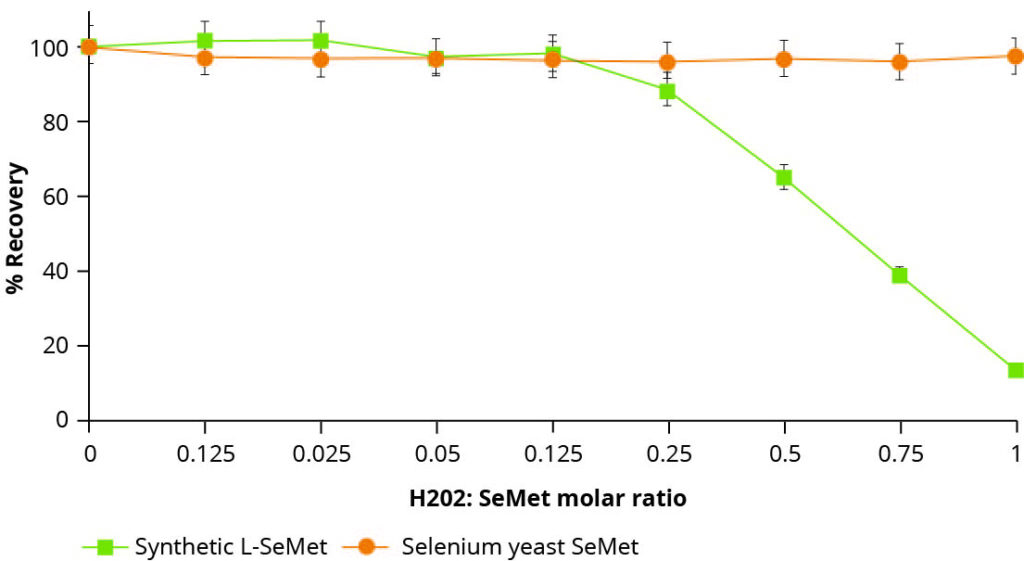
These results are illustrated in Figure 2, whereby, upon increasing the oxidising conditions of the solution, the recovery of synthetic L-selenomethionine in its pure form rapidly declines. In contrast, recovery of selenomethionine from the organic selenium yeast remained constant under the same conditions, thus illustrating its greater stability and reduced susceptibility to oxidation. While not a direct in vivo measurement, this simple technique can usefully indicate the potential stability or instability of trace element forms in an oxidising environment.
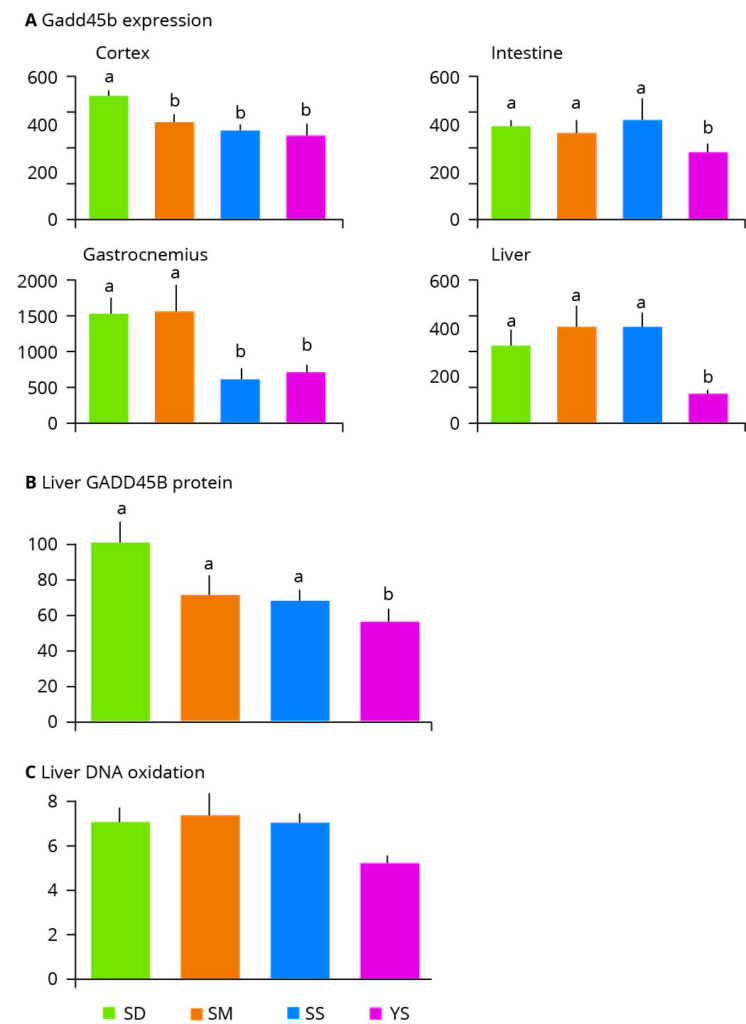
Figure 3 – Effect of selenium supplementation on markers of DNA damage. SD: selenium deficient, SM: chemically synthesized Se. SS: sodium selenite, YS: Selenium yeast. Values with different letters indicate statistically significant differences (P<0.05).
Anti-oxidant vs. pro-oxidant
The main purpose of adding selenium to a diet is to protect against oxidative stress. The primary result of oxidative, or oxygen radical-induced, stress is DNA damage. Research which assessed the differential effects of sodium selenite, synthetic L-selenomethionine and selenium yeast on gene expression in an animal model, produced some notable findings. The authors examined the induction of genes and proteins linked to DNA damage in addition to physical markers of oxidative stress (Figure 3). They found that expression of the DNA damage response gene Gadd45b was consistently lowered its expression in all tissues following organic selenium yeast supplementation (Figure 3A). Inorganic sodium selenite reduced Gadd45b expression in the cortex and gastrocnemius and the synthetic selenium source was limited in providing cellular benefits and only reduced its expression in the cortex (Figure 3A).
The authors also observed that in the liver, selenium yeast significantly reduced the abundance of the protein encoded by the Gadd45b gene, demonstrating the protective impact of selenium yeast and contrasting with the inability of inorganic and synthesised selenium sources to do so (Figure 3B). Additionally, the authors measured the levels of a marker of oxidative DNA damage (8-oxo-dG) in the liver and found a 27% reduction in 8-oxo-dG in the selenium yeast supplemented animals, again indicating the protective benefits of selenium yeast (Figure 3C).
Figure 4 – p53 tumour oncogene expression SD: selenium deficient. SM: chemically synthesized Se. SS: sodium selenite. YS: Selenium yeast.
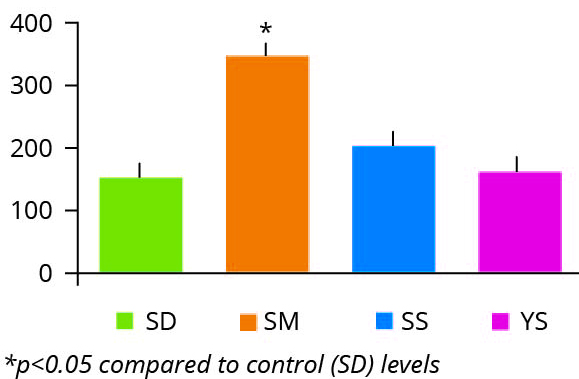
Perhaps the most surprising additional finding of this study was the marked induction of the p53 gene in the intestinal tissue of animals fed diets containing the chemically synthesised selenium source (Figure 4).
It is well documented that the tumour suppressor gene p53, a transcription factor that controls cellular response to DNA damage, is induced in the acute cellular response to DNA damage and also in response to chronic, increased damage observed in ageing tissues. Therefore, the level of expression of p53 serves as a gauge of the endogenous levels of genomic instability. This suggests that the synthetic selenium source, unlike selenium yeast, promotes intestinal genotoxicity, potentially a reflection of the acute oral toxicity data highlighted in Table 2.
In the context of the overall data, it is clear that selenoamino acid and selenoprotein bioavailability will vary between sources as they transit the GI tract. At a more simplistic level, differences in the stability of selenium sources can also be expected to impact parameters such as shelf life and product quality.
More importantly, such differences may have a bearing on the toxic potential of different preparations and, in the case of chemically synthesised selenium sources, impart similar toxicity to that of inorganic sodium selenite.
Conclusions
- Selenium source differences have implications for product performance ranging from shelf life and toxicology to animal response.
- Clear differences in the relative stabilities of selenium products can be demonstrated at the product, premix and feed level.
- When compared to selenium yeast, chemically synthesised selenomethionine was relatively unstable and had limited cellular potential in terms of promoting an antioxidant response.
- Antioxidant functioning can be compromised after exposure to high levels of chemically synthesised selenium.
- This can result in altered gene expression patterns leading to oxidative stress and alterations of cellular function, in addition to both cytotoxicity and proteotoxicity.
References available upon request.











