Understanding the manganese pathways

Farm productivity, animal welfare and the quality of animal products depend on various nutritional factors, including dietary trace elements. Manganese (Mn) is one of these essential minerals. It plays a role in oxidative defence, contributes to skeletal development, and has an effect on reproductive hormones.
Manganese is involved in many biological functions, such as the urea cycle and the Krebs cycle, through the Mn-dependent enzymes, arginase and pyruvate carboxylase. It can also reduce oxidative stress via the activity of the MnSOD. In livestock and poultry, Mn supplementation can improve the quality of bones, the quality of the eggshell and support reproductive performance. Requirements are around 4 ppm for piglets, 20 ppm for sows and dairy cows, 25 ppm for laying hens, and 60 ppm for broilers and turkeys, according to NRC.
In general, generous safety margins are applied by feed companies, with supplementation levels well above the physiological requirements. The maximum dietary concentration in the European Union is 150 ppm for all species, except for fish at 100 ppm. Manganese, like other metallic compounds, is a non-renewable mineral resource, and depletion of global reserves drives the search for more sustainable practices. In order to optimise the supplementation of Mn, it is important to understand the fate of this trace element in the body, and its interactions with other nutrients.
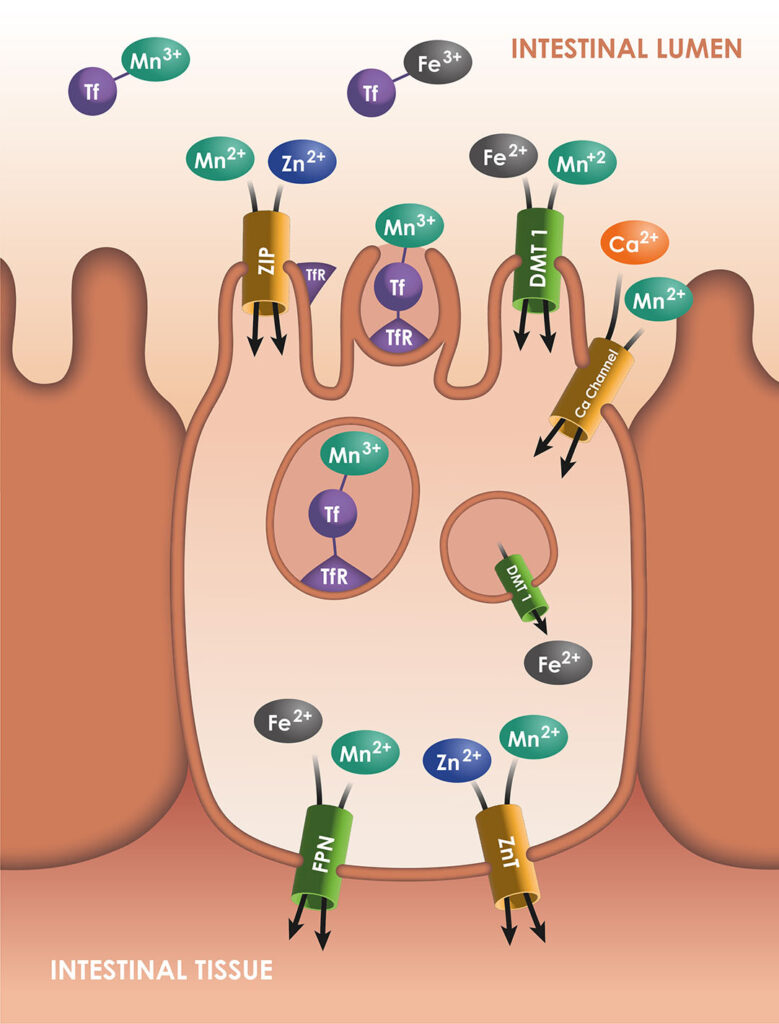
Mn transporters
Mn is a transition element. Its typical properties are very similar to the properties of iron (Fe), another transition metal. These elements have multiple oxidation states and are redox-active. For dietary Mn, the main oxidation state is Mn+2, which is water-soluble like Fe2+.
Mn concentration in the organism is stable in standard conditions due to the homeostatic regulation of this element, with a strict balance between intestinal absorption and excretion. Some essential elements such as zinc (Zn) and copper (Cu) are absorbed into the intestinal cells by specific transporters. For Mn, some transporters have been discovered, but they were previously identified as transporters of other metals.
As Mn2+, Mn is soluble and available for the Divalent Metal Transporter 1 (DMT1), which is a major transporter for Fe2+ and some other cations. Its transporter is considered to be the main transporter of Mn. In a study performed with DMT1 knockout rats, the Mn absorption was reduced by 70%. Similarly, ferroportin (FPN) is known as an exporter of Fe2+, but could also export Mn2+. Some transporters of Zn increase the Mn concentration in the intestinal cells (ZIP8 and ZIP14, which could also transport Fe), while ZnT10 is involved in the Mn efflux to the blood. In addition, Fe2+ and Zn2+ are not the only cations sharing their transporters with Mn: the ions Mn2+ can enter in the enterocytes through the voltage -regulated and ionotropic glutamate receptor Ca+2 channels.
Mn and Fe can also circulate in the organism under the oxidation state of 3+. They precipitate, as they are insoluble, unless they are bound to ligands. When they are bound to transferrin, they are imported into the cell through receptor-mediated endocytosis, via the transferrin-receptor TfR. In any case, Fe2+ and potentially Mn2+ would be oxidised as Fe3+ and Mn3+ by ceruloplasmin, before entering the blood stream, and then transported by transferrin.
Mn absorption according to the Fe status
As Mn and Fe share the same transporters, an interaction between these elements is recorded in some studies. Mn supplementation could increase the gene expression of Fe transporters (DMT1 and FPN), and Mn transport is impacted by the Fe status, especially by Fe deficiency. When dietary Fe is low, the expression of DMT1 increases, leading to increasing concentrations of Mn in various tissues such as brain, kidney and bones. However, an excess of Fe could decrease the Mn concentration in the liver or in the intestine, but an expression of Fe transporters such as DMT1 and FPN would not be impacted. Fe2+ and Mn2+ could compete for uptake by Fe transporters.
Conclusion
Mn, which is predominantly absorbed under the oxidation states 2+ and 3+ (Mn2+ and Mn3+), enters into the cells through different transporters, which are associated with other cations (Fe2+, Zn2+, Ca2+). Consequently, its absorption depends on the other metals, and especially on the Fe status. Optimising Mn supplementation and understanding the Mn fate in the organism are consequently correlated with a better understanding of mineral interactions and better knowledge of feed composition.
This article is one of a series of articles summarising the absorption pathways of trace minerals including zinc (AAF Vol 22 No. 7 2014) and copper (AAF Vol 25 No. 3 2017).


