The intestine: an overlooked aspect of mycotoxin challenge
When considering mycotoxins, the focus is normally only on the post absorptive effects of mycotoxins, whether they manifest in chronic or acute signs. Leon Broom and Murray Hyden from Anpario explain why the impact of mycotoxins on the intestines is just as important.
Fungal biochemical pathways can yield various compounds that are not considered to be necessary for their growth and thus are referred to as secondary metabolites. These compounds have been found to have wide ranging biological effects and include potent poisons (mycotoxins). Fungi frequently infect crops and the subsequent contamination with mycotoxins poses a significant health risk to both humans and animals consuming the crop material. At the cellular level, toxins can catastrophically interfere with numerous pathways and processes. Some better understood mycotoxins are known to inhibit protein synthesis (e.g. Creppy, 2002). Therefore, cells that are rapidly multiplying or synthesising/secreting protein(s) (eg intestinal, immune, etc.) would be particularly vulnerable to the effects of mycotoxins.
Intestinal environment
When considering mycotoxins, the focus is normally only on the post absorptive effects of mycotoxins, whether they manifest in chronic or acute signs. We, however, believe that this is a major oversight of the impact mycotoxins have in the intestinal environment. The gastrointestinal tract (GIT) is the initial site for interaction of ingested mycotoxins with the animal. Mycotoxins have varying bio-availabilities. Some will be more rapidly absorbed, whilst others will get further along the GIT. This is very important for a number of reasons. Firstly, whether absorbed into the systemic circulation or not, the cells of the GIT will potentially be exposed to the full range of ingested mycotoxins and in the highest concentrations. Secondly, toxins that get further along the GIT will have had more opportunity to interact with the microbial cells present in the intestine. These cells can also be vulnerable to the effects of mycotoxins.
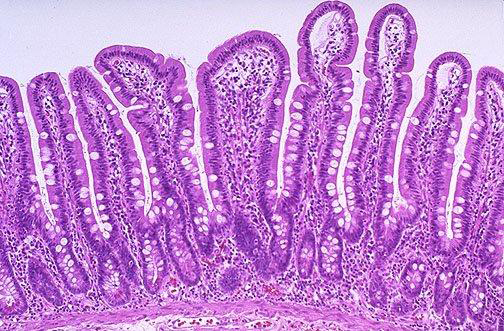
Viability of the intestinal cells
Recent work by Alassane-Kpembi and others (2013) studied the effects of the Type B trichothecenes on intestinal epithelial cells. They demonstrated that these mycotoxins have a negative effect on the viability of the intestinal cells. When discussing feed mycotoxins, low concentrations are often dismissed as being of very little significance. We strongly reject this notion. The work of Alassane-Kpembi and others supports our view, as the effects on cell viability, per unit of mycotoxin, were much greater at the lower concentrations than higher ones. They also reported that in almost all cases, the effects of combinations of the toxins were either additive or indeed synergistic, reinforcing the fact that it’s completely inappropriate to consider any single mycotoxin in isolation. Obviously, the importance of the viability of intestinal cells in maintaining the performance and health of the animal cannot be overstated.
The intestinal microbiota also plays a crucial role in determining the health and performance of the animal. An optimal microbiota prevents colonisation of the intestinal epithelium by pathogens and penetration of the gut barrier, modulates the gut-associated lymphoid tissue (GALT) and systemic immunity, and influences gastrointestinal development. The combined effects are better digestive efficiency and utilisation of nutrients. It goes without saying that microbial cells can be susceptible to mycotoxins. Recent work by Ouethrani and others (2013) demonstrated that ochratoxin A significantly reduced acetic, butyric and total short chain fatty acid concentrations in a dynamic simulation model of the descending human colon. For acetic acid, butyric acid and total SCFA’s the differences were significant. This would indicate that ochratoxin A is able to affect the composition and/or metabolism of the colonic microbiota. Moreover, and in support of this, the work of Ouethrani and others revealed that ochratoxin A eliminated a strain of Lactobacillus reuteri from the descending colon microbiota, which was permanent. L. reuteri, which produces the bacteriocin reuterein, can be a key resident of the gastrointestinal tract, having been shown to have positive effects on intestinal disorders, infection and immune responses. Clearly, mycotoxins can influence the composition and fermentation products of the intestinal microbiota and, in doing so, affect the health and performance of the animal.
Multitude of mycotoxin metabolites
With all of this in mind, we must now consider the array of mycotoxins that can be present. Currently, there are some 300-400 known mycotoxins. Recent work analysing 83 feed and feed raw materials revealed that all of the samples contained a multitude (7 to 69) of mycotoxin metabolites (Streit and others 2013). The most samples contained 26-30 different metabolites. But also some samples even had more than 50 different metabolites in them. The total number of different metabolites that were detected was 139. It is, therefore, more than likely that any given feed raw material or finished feed is contaminated with a multitude of mycotoxins and, as our understanding of the diverse array of mycotoxins develops, it may well become apparent that our focus on the current 6 ‘main’ mycotoxins (aflatoxin, ochratoxin, zearalenone, fumonisin, deoxynivalenol and T2) has been misplaced.
Synergistic effects
At this point in time, this rapidly developing area is generating more questions than are being answered. This potentially puts scientists working in this area and companies seeking to find solutions to mycotoxins for animal production in an uncomfortable position. We must, however, be honest about the current situation – there are a multitude of mycotoxin metabolites contaminating feed raw materials, plant material used as bedding and conserved forages and we barely understand the full impact that the ‘main’ mycotoxins that have been studied have on human and animal health and performance. Moreover, mycotoxins do not occur in isolation and their effects seem likely to be synergistic or at least additive. Whilst it is now possible to have samples analysed for numerous mycotoxins, only partial interpretation of what this actually means to animal (or human) health and performance is currently possible. However, not fully understanding the mycotoxin situation does not mean that we ignore this potentially considerable challenge to animal production. There are products available that help to alleviate the mycotoxin challenge to the animal. Currently, it would be advisable to use a product with the broadest efficacy against tested mycotoxins (based on economic and scientific evaluation). Broader efficacy will remove more mycotoxins from the toxicological ‘equation’, whether they are the typically tested or emerging mycotoxins with similar physical or chemical characteristics. Fungi are proposed to be the greatest threat to animal and plant health of all the taxonomic classes of pathogens (Fisher and others 2012). We believe that we’re only just beginning to appreciate the magnitude of the challenge fungi/mycotoxins present to the animal and maximising performance.
References are available on request











