Pure bis-glycinates: Highly available trace minerals
Most trace minerals are added to feed in the form of inorganic salts: oxides or sulphates. However, these inorganic sources are known to have low availability. Large quantities pass through the animal and end up in the manure because they have not been absorbed by the intestines. We therefore need sources of higher available trace minerals, such as chelates based on synthetic glycine.
By Sjo Zwart , Tessenderlo Chemie, Aliphos division, the Netherlands
Current legislation has lowered the use of inorganic minerals towards nutritional needs (2003). There are indications that these levels will be reviewed critically again in the short term in order to reduce the output of trace metals in the environment. This will most likely result in the levels being lowered even further. There is therefore an urgent need for sources of higher available trace minerals. The use of metal-chelates based on soy-derived protein is allowed for longer within the EU as a source of trace minerals, assuming a higher availability for such products and thus coping with lower inclusions rates. Since 2006, a special type of chelate – glycinate (based on synthetic glycine) – is allowed to be used as a source of trace minerals*.
Glycine chelates
In the early 1980s Tessenderlo Chemie Belgium acquired the technology for the production of glycine. Shortly after new products were developed that were derived from or based on glycine. Since then, the company has enlarged its portfolio to more than 40 different derivatives based on glycine. In 2007, the company began the production of metal bis-glycinates** (hereafter called AG) consisting of a copper, manganese, zinc and ferrous bis-glycinate (Figure 1).AG is based on synthetic glycine and contains two molecules of glycine per metal ion. The copper, manganese and zinc product of AG do not contain other ions like sulphate. However, the commercial ferrous product is a ferrous-glycine-sulphate complex.
Analytical methods
Up until now it has been difficult or even impossible to define the structure of metal chelates based on, for example, soy-derived protein. This is, of course, a prerequisite since real metal proteinates are assumed to have higher metal availability for the animal. On the contrary, chelates based on glycine can be defined using several analytical methods, starting with the simple wet chemical methods.
A chemical pure bis-glycinate of zinc, for example, contains 30.6% zinc and 13.1% nitrogen in a ratio of 2.34 without the presence of other ions. The product AG zinc contains a minimum of 30% zinc, 12.7% nitrogen, and some residual water (<1%), which guarantees the presence of the chemical pure product (with a slight excess of glycineto ensure complete conversion).
It can be shown that AG zinc has the necessary metal:glycine ratio of 1:2, and is stoechiometrically a pure Zn-glycinate. This is not the case with most other so-called glycinates on the market, which mostly contain important quantities of sulphate, sometimes up to 40%, and as a consequence have much lower metal and nitrogen levels. Moreover, those are different chemical entities and might behave differently in the intestine.
Product purity
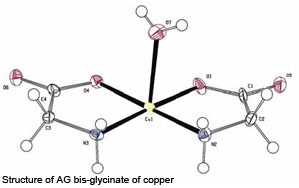
In addition to basic analytical methods, other more sophisticated methods exist to define the molecular structure of metal bis-glycinates. One of these methods is the so-called 13C solid state NMR spectroscopy. This technique enables us to determine whether or not the product is a real bis-glycinate. When such a spectrum is made of glycine, the two C-atoms clearly stand out. A spectrum of AG zinc looks a bit different, because the carbonyl absorption turned from a singlet into a multiplet due to chemical interaction of this carbon with the zinc ion – a positive indication that the pure zinc bis-glycinate is present. Going one step further, single crystal X-ray diffraction is acknowledged to be the analytical method for ultimate structure determination. As an example, the structure of AG bis-glycinate of copper was determined after crystal-lisation (hence the molecule of water in this picture) using this method.
Infrared analysis
Both analytical techniques are likely not within reach for most of our customers. Therefore, Tessenderlo Chemie has developed a simple and reliable method to identify whether a product is a real bis-glycinate based on infrared spectra analysis. It is widely accepted (e.g. by the FDA) that if the infrared spectrum of an “unknown” is identical with the infrared spectrum of a “standard”, that both molecules are identical. This means that if infrared of a commercial “bis-glycinate” is identical to the infrared spectrum of a standard, one has proven his case. When this analysis is performed on a pure zinc bis-glycinate, the “carbonyl stretch” at approx. 1600 cm-1 wavelength is clearly shown. The infrared-spectra of AG zinc also clearly shows this carbonyl stretch. Infrared analysis was also performed using products from the competition, and it was noticed that all samples that were analysed showed a wide and intense absorption at wave number 1150 cm-1, implying the presence of SO4. In addition, less intense carbonyl absorption at a wave number of 1600 cm-1 is also shown. This offers further evidence that metal bis-glycinates from other sources are not pure metal bis-glycinates, but some are merely mixtures of metal and glycine. In conclusion, contrary to other forms of chelates, the purity and structure of bis-glycinates can be proven using different fundamental analytical methods and techniques, including infrared-spectra analysis.
* Regulation 479/2006 authorises the use of chelated form of copper, manganese, and zinc with synthetic glycine as a feed additive. Using copper glycinates as an example, the products are defined as: Cu(x)1-3.nH2O where x is the anion of synthetic glycine.
** AliGlys® (marketed by Aliphos®, a division of Tessenderlo Chemie)
Source: AllAboutFeed vol 1 nr 3, 2010











