The often-overlooked danger of fumonisins

Fumonisins are mycotoxins produced by Fusarium fungi, commonly found in contaminated grains used in animal feed. While they are well-known for posing a serious risk to human health, their impact on livestock is often overlooked. Given the widespread presence of fumonisins in animal feed, it is essential to raise awareness and implement effective monitoring and management strategies to protect animal health.
MYCOTOXINS 2025: Utilising technology to detect & mitigate – read all articles
Global climate change increases the occurrence of the mycotoxin fumonisin through stress factors like high temperatures, drought, floods, and mechanical damage to plants (e.g., harvesting, insect infestation, diseases). These factors promote fungal growth, especially of Fusarium verticillioides and Fusarium proliferatum, which thrive in stressed plants. Fusarium fungi are particularly harmful to maize, where they survive in small plant parts and kernels. Fumonisin, a byproduct of the fungus, damages plant cell walls and membranes, disrupting metabolism and impairing nutrient and water transport, leading to reduced plant health.
What does fumonisin do in the animal body?
Fumonisin’s toxic effect disrupts sphingolipid metabolism. Sphingolipids, crucial for cell membrane structure, signal transduction, cell communication, and immune response, are synthesised through enzymatic reactions and concentrated in the outer layer of membranes, where they interact with other lipids and proteins.
Sphingolipid production begins in the endoplasmic reticulum of the cell. Initially, sphinganine (SA) is formed, which serves as a molecule for the further sphingolipid compounds. SA is then converted into ceramide by the enzyme ceramide synthase. Ceramide is an important intermediate in sphingolipid metabolism, as it forms the basis to produce various complex sphingolipids.
Ceramide is then transported to the Golgi apparatus, where it is converted into various sphingolipids, including sphingomyelin and glycosphingolipids. The modified sphingolipids are then packaged into vesicles and transported to their destination, such as the cell membrane.
In the cell membrane ceramide is converted to sphingosine (SO), which is a crucial intermediate in sphingolipid metabolism. Then SO undergoes additional modifications, including its conversion into sphingosine-1-phosphate (S1P) by sphingosine kinase. S1P is a key regulatory lipid metabolite, central to cell migration and immune response. It helps T-cells and other immune cells migrate from the lymph nodes into the bloodstream. This is important for immune responses and inflammation regulation. Moreover, S1P controls cell movement, which is crucial for processes such as wound healing and immune system development.
Figure 1- Sphingolipid metabolism and its disruption by Fumonisins.
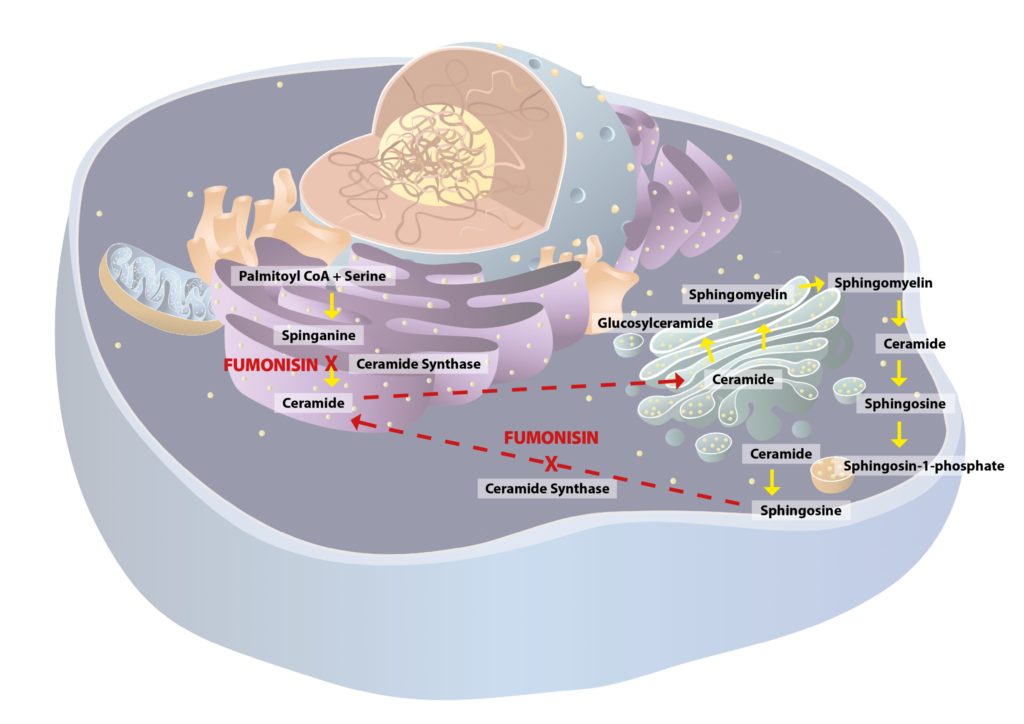
Figure 1 shows fumonisin inhibits the enzyme ceramide synthase, which is necessary for ceramide production. This results in the accumulation of SA, and to a lesser extent SO, in cells, blood, and urine. The accumulation of SA and the lack of sphingomyelin can disrupt normal cell function. At high concentrations, SA are toxic. They impair the structure and function of cell membranes and can lead to cell damage or death. Cells in organs such as the liver and kidneys, which are responsible for detoxification and metabolism, are particularly vulnerable.
What does fumonisin contamination mean for livestock production in practice?
Reduced vaccination effectiveness:
S1P is an important regulator of immune response and cell migration. Fumonisin disrupts the formation of S1P, impairing the body’s ability to mobilise immune cells and respond to infections. This can lead to weakened immune defence and increased susceptibility to infections, thus raising the risk of disease and inflammation. The molecule S1P also plays a key role in the movement of T-cells and other immune cells from the lymph nodes into the bloodstream and to infection sites. Reduced immune cell migration impairs the body’s ability to respond to antigens (such as components of a vaccine). Immune cells cannot effectively reach the infection area to coordinate the immune response. Additionally, B-cells, responsible for antibody production, may also be impaired by fumonisin, affecting the function of the vaccine. As vaccinations often aim to stimulate antibody production, reduced B-cell activity results in lower antibody formation, reducing the effectiveness of the vaccine.
Gastrointestinal Inflammation:
Sphingolipids, especially sphingomyelin, are essential components of cell membranes in all body cells, including epithelial cells in the gastrointestinal tract. By inhibiting the enzyme ceramide synthase, SA accumulates, and sphingomyelin is reduced in cell membranes. These changes impair the stability of cell membranes in the gastrointestinal tract, negatively affecting the barrier function. Additionally, altered epithelial cells disrupt nutrient transport mechanisms in the gut cells, leading to impaired nutrient absorption.
Reduced performance:
Fumonisin-induced disruption of cell functions can result in a reduced production of digestive enzymes, slowing down and making the digestion process less efficient. Enzymes that can be affected include amylases, proteases, and lipases, which are responsible for breaking down carbohydrates, proteins, and fats. This results in performance losses as well.
Compromised gut health:
The destruction of epithelial cells in the gastrointestinal tract and the impairment of immune response can disturb the gut microbiota. The microbiota plays a key role in digestion and maintaining gut health. An imbalance in the microbiota can lead to inflammation of the mucosa and digestive issues.
Standard use of mycotoxin-deactivating products from the MiaBond line is recommended to prevent subclinical stress, support nutrient uptake, and maintain microbiota balance. A new approach involves using MiaBond Drink via the water supply, offering flexibility in responding to fumonsin presence in feed. This combination of toxin-inactivation, inflammation reduction, gastrointestinal health support, and immune support promotes animal performance.
References are available on request.







