Challenges in measuring and minimising mycotoxins

Mycotoxins are toxic fungal metabolites which occur naturally in major cereal constituents as well as minor ingredients of animal feed. Testing animal feed with ingredients coming from all corners of the globe requires multi-mycotoxin monitoring.
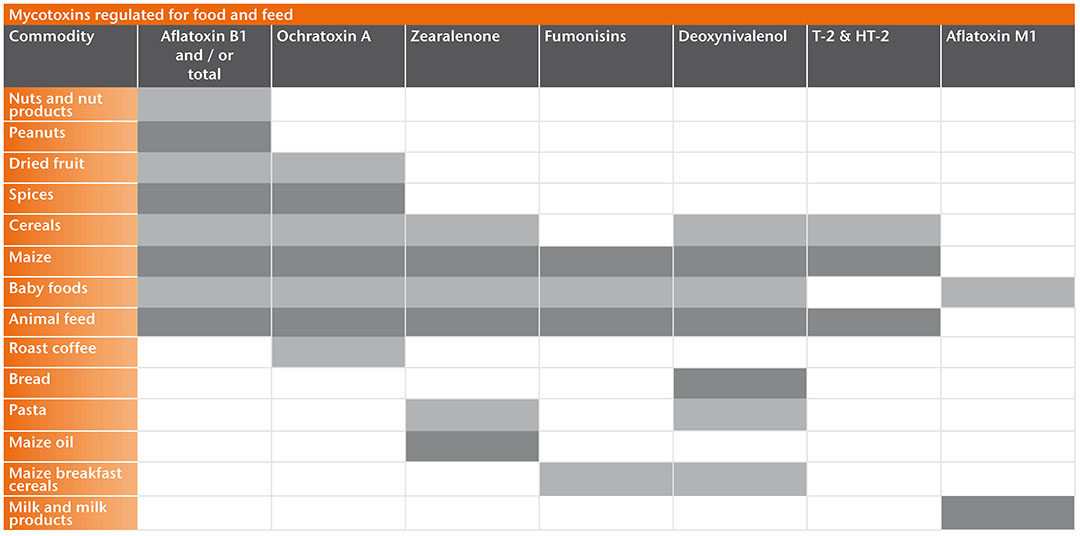
Different components of animal feed are liable to contain a variety of different mycotoxins. Aflatoxin B1 is the only regulated mycotoxin with an EU limit but there are guidance values for levels of deoxynivalenol (DON), zearalenone (ZON), ochratoxin A (OTA), fumonisins B1 and B2, T-2 and HT-2 toxins. Monitoring these mycotoxins in feed components and finished products is essential.
Identifying potential risk for human consumption
The risk assessment for mycotoxins in feed identifies firstly the threshold level at which adverse health effects can affect the animal and secondly whether the mycotoxin or any metabolite can transfer to animal products and present a risk for human consumption. The levels at which adverse health effects can occur in animals is very much species dependant. For example, for some mycotoxins such as OTA and ZON pigs exhibit high sensitivity, whilst poultry can tolerate much higher levels without showing adverse effects. Horses are extremely susceptible to the harmful effects of fumonisins. Regulations controlling mycotoxins in animal feed therefore relate to the intended use of the feed, with different limits being applied for different animal species.
Routine mycotoxin testing
Although several mycotoxins can be transferred to animal products intended for human consumption only aflatoxin M1 in milk being a metabolite from aflatoxin B1 in feed for dairy cattle is a major concern (see image below). Aflatoxin B1 is therefore strictly controlled in dairy cattle feed. OTA can be transferred to meat and offal and this is more of a specific concern in some countries where regulations are in place.
Another concern related to the occurrence of mycotoxins in animal feed is not directly addressed in risk assessment, and that is the potential impact on animal productivity. For example, small effects on yields such as weight gain or egg production may be evident from exposure of poultry to low levels of mycotoxins from feed with important economic consequences for farmers. There is therefore a strong motivation to minimise occurrence of mycotoxins in animal feed and routine testing plays an essential role.
Feed ingredients from around the globe
Mycotoxin guidance values are multi-layered with limits applied both to feed components as well as the finished products means that levels of contamination need to be determined for both raw materials to ensure compliance, as well as an aid to mixing of compound feeds. The multitude of ingredients used in animal feed from diverse geographical origins necessitates monitoring all the mycotoxins of potential concern and testing of animal feed requires multi-mycotoxin monitoring.
‘Dilute and shoot’ approach
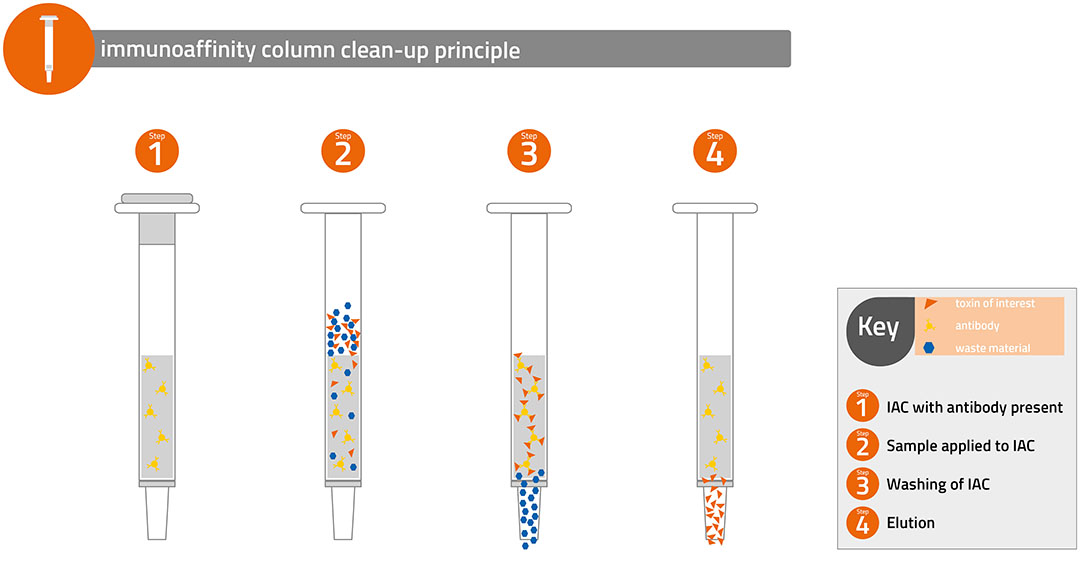
There are many multi-toxin methods employing mass spectrometry that advocate a ‘dilute and shoot’ approach. This involves extracting a sample, filtering and directly analysing. It is however recognised that this approach is affected by co-extractives from the feed matrix and measures are essential to correct for ‘matrix effects’. These measures often include matrix matched calibration and the use of expensive stable isotope-labelled internal standards. This simplistic approach to feed analysis is essentially a screening method and for accurate quantification and in particular analysis of complex feed components such as distillers dried grain solubles it is essential to conduct a full clean-up of the feed extract before analysis (see image below).
The most reliable and effective clean-up is achieved using an immunoaffinity column (IAC) when an extract of the sample is directly passed through the IAC and the targeted toxins selectively retained . Suitable IAC should be designed for the simultaneous analysis of all regulated mycotoxins that can occur in animal feed and demonstrate high recoveries and good precision thereby satisfying the method performance requirements for analytical methods.
Gold standard method
It has been previously shown that instrumental analysis of samples with no clean-up is adequate for screening, but for definitive measurements IAC clean-up is essential. These interferences which impacted on quantification were not present after IAC clean-up. Whilst for surveys conducted for academic purposes a ‘rough and ready’ quantification might be acceptable, in the animal feed sector uncertainties around compliance limits cannot be tolerated. Gold standard methods employing IAC clean-up are essential to adequately assess risks from mycotoxins in animal feed. This is the case whether the analysis is conducted in-house or externally by contract or official laboratories.











