Beat the inflammation for full potential
The immunological processes, and more particularly inflammation, can be a high cost for animal growth, hence leading to less efficient production. However, by supplying anti-inflammatory compounds we can counteract these negative effects.
Inflammation is defined as a reaction of tissue to any harmful stimulus. This stimulus can either be of physical, chemical or immunological origin, but can also be caused by microorganisms like bacteria, parasites, and viruses. As the gastrointestinal tract (GIT) is the largest immune organ of the body and the place is where 70% of the immune cells are located, these challenges will often result in the activation of the gastrointestinal immune (GI) system. Hence, specialised cells start to do their job, especially pro-inflammatory cytokines (e.g. interleukin-6 (IL-6)). Cytokines are polypeptides produced mainly by monocytes, macrophages, and lymphocytes and regulate the intensity and duration of an immune response. It is known that even mild immune challenges already induce a reduction in feed intake and consequently can have a negative impact on growth. In addition, the immune cells require nutrients and energy to perform their function.
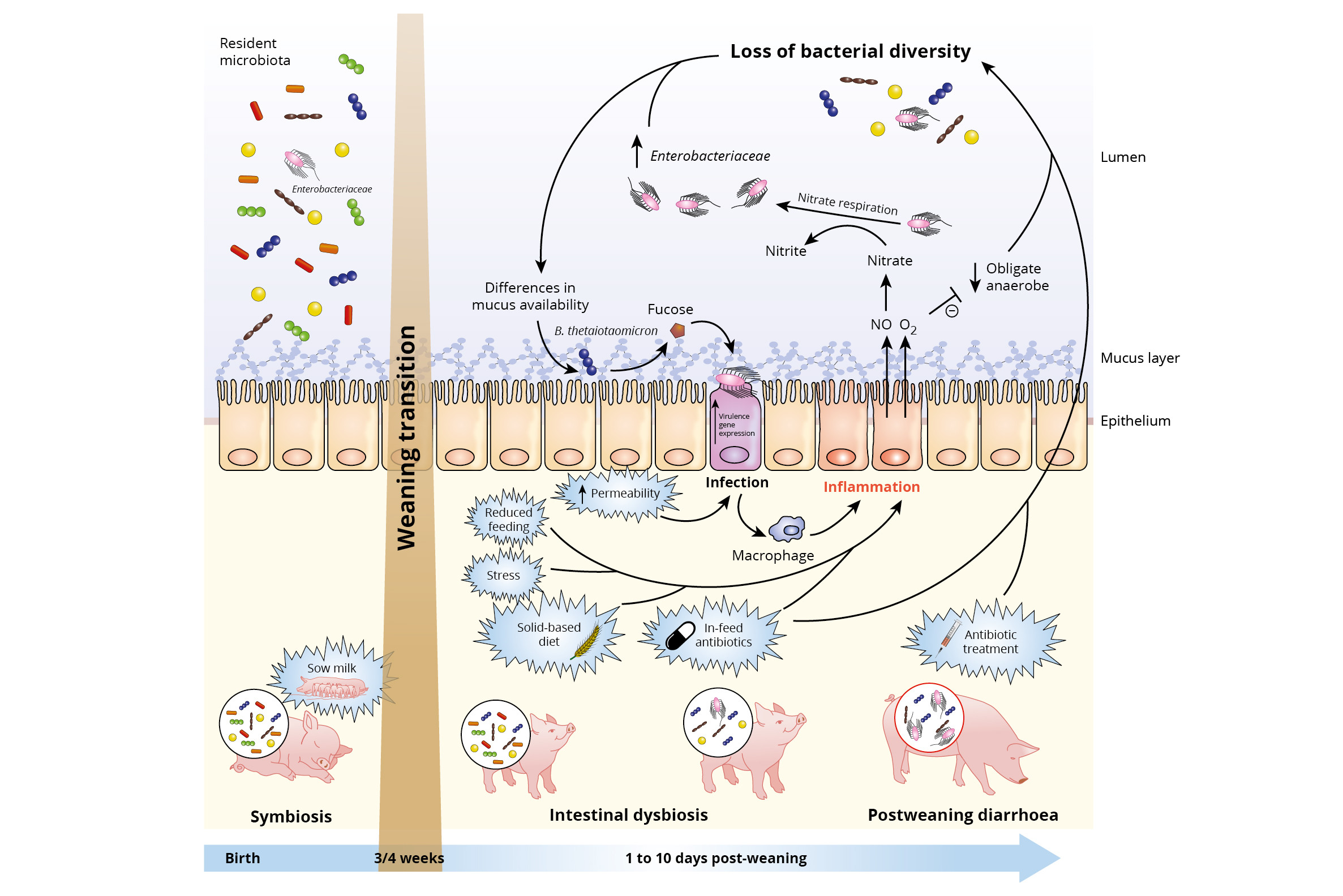
Figure 1 – Impact of weaning transition on piglet gut microbiota and expansion of infectious agents. Reprinted from Cell Press Reviews, Trends in Microbiology, vol. 25, no.10, Raphaële Gresse, Frédérique Chaucheyras-Durand, Mickaël Alain Fleury, Tom Van de Wiele, EvelyneForano, and Stéphanie Blanquet-Diot, Review – Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health, 851-873, Copyright 2018, with permission from Elsevier.

There is also an increased protein demand, because of protein requirement for the production of acute-phase proteins and antibodies. Processes as lipolysis, proteolysis and glycolysis (degradation of fat, protein and glucose respectively) will provide the required nutrients and energy for the induced immune response. However, due to the reduced feed intake, body reserves may be used to release the required nutrients and energy. In case of a severe or prolonged infection this will result in a clear loss of body weight. For instance in humans, a severe infection can lead to a loss in body weight of 15-30%.
Weaning impacts GI immune system
The moment of weaning results in three major changes for the pig. First of all the diet composition is changed drastically from easily digested milk to a less digestible solid-based diet. Secondly the pigs are moved to a new environment. And thirdly, litters are mixed which brings social stress to the animal. All these factors have a major impact on the piglets’ health and performance and might sometimes even result in mortality. The change in diet composition might have a huge impact on their feed and water intake, which results in a fasting period of 24 hours or even up to 48 hours. For sure, this has a negative effect on the growth rate of the weaned piglet.
However, the change in diet composition has also a huge impact on the intestinal microflora. The colonisation of the gut microbiota is developed based on the sows’ milk and also favours the digestion of milk. At the moment of weaning, the gut microflora will be adapted to the new solid-based diet composition. Before the dietary change, the piglets’ microflora is well balanced and is referred to a state of symbiosis or eubiosis. However, the changes and disturbance of the bacterial gut population induced by the dietary change will result in a state of dysbiosis. When a piglet is in a status of dysbiosis, there is an increased risk for post weaning diarrhoea. Moreover, weaning is associated with increased permeability of the intestinal epithelium, which increases the risk of pathogenic infections as it becomes easier for pathogens to cross the epithelium and enter the body. Gresse et al. (2017) summarised the hypothesised underlying mechanisms of inducing dysbiosis in weaned piglets (Figure 1). The reduced feed intake at weaning, the change from milk to a less digestible solid-based diet, stress induced by social and environmental changes, but also the use of in-feed antibiotics, increase the risk of local intestinal inflammation. The latter is explained by the fact that in-feed antibiotics might affect both pathogenic and non-pathogenic bacteria and consequently lower the bacterial diversity and increasing the risk of inflammation caused by pathogens. When an inflammation is induced, macrophages produce Nitric Oxide (NO), which has some antibacterial properties. However, in the lumen NO is rapidly transformed to nitrate.
A nitrate rich environment is beneficial for strains like E. coli, but non-favourable for the obligate anaerobic bacteria like Clostridia and Bacteriodia. Hence, the balance between the good (Clostridia and Bacteriodia species) and the bad bacteria (E. coli) is disturbed. In addition, inflammation increases the oxygen level in the lumen, which also results in a reduction of the obligate anaerobic bacteria and consequently an increased loss of bacterial diversity. This creates more opportunities for the pathogenic bacteria to degrade the mucus polysaccharides into sugars, for instance fructose. This specific sugar is used by e.g. enterohaemorrhagic E. coli to activate the type III secretion system (T3SS) gene expression, which is used to adhere to the gut enterocytes and cause an infection. Again macrophages are activated and NO will be produced, lowering the bacterial diversity and everything starts all over again. Hence, we have to find a way how we can breach this vicious circle.
Figure 2 – Effect of 3 different compounds on NO production induced by LPS challenge.

How to find anti-inflammatory compounds?
It is important to remember that the GI immune system should be activated in case of high pathogenic pressure, but we should avoid that the immune system over-reacts when small challenges occur. If we can manage to reduce the negative impact of pro-inflammatory processes by supplying anti-inflammatory compounds, we are one step closer to achieving 100% of the genetic growth potential of an animal. At the laboratory of Prof. Niewold at KU Leuven in Belgium, an in vitro model is available to study the potential anti-inflammatory effects of compounds. FRAmelco requested to use this model to study whether alpha-monolaurin and a combination of alpha-monocaprylin and alpha-monocaprin have anti-inflammatory properties. In this model, macrophage-like cells are used and are challenged with lipopolysaccharides (LPS). Upon this challenge, the macrophage-like cells will produce NO. When the selected compound is able to reduce NO production, it means that the compound has anti-inflammatory properties. The inhibitory concentration 50 (IC50) indicates from which dose level onwards an anti-inflammatory effect is observed. Oxytetracycline (OTC) was used as a control group and from Figure 2 it became clear that OTC showed the expected reduction in NO production at IC50 of 400 ppm. Alpha-monolaurin also showed a clear reduction of NO production, but already at IC50 of 200 ppm. The combination of alpha-monocaprylin and alpha-monocaprin showed intermediate results with an IC50 of 800 ppm. Based on these results it can be expected that feed additives based on alpha-monolaurin, alpha-monocaprylin and alpha-monocaprin have anti-inflammatory properties. In vivo trials should confirm these expected effects.
Author:
Olga Dansen, correspondent, FRAmelco, the Netherlands











