Organic trace minerals: Selecting the right source

To get the most nutritional and economic value from a feed formulation, nutritionists need to pay special attention to what they are using, especially when it comes to “organic” trace mineral sources. This white paper surveys all considerable aspects when selecting a suitable trace mineral source.
The chemistry of complexation or chelation, as it is commonly known, has created a great deal of confusion in the animal feed industry. Terms such as metal amino acid complexes, metal amino acid chelates, metal polysaccharide complexes and metal proteinates abound, yet official definitions remain vague and unhelpful. Organic trace minerals (OTMs) are recognised as a more bioavailable source of trace elements than their more traditional inorganic counterparts, such as sulphates and oxides. But official terminologies provided by the Association of American Feed Control Officials (AAFCO) and the European Food Safety Authority (EFSA) on what constitutes an “organic” trace mineral can be ambiguous.
Typically speaking, chelates are prepared by reacting inorganic mineral salts with, for example, enzymatically prepared mixtures of amino acids and small peptides under controlled conditions. Such amino acid and peptide ligands bind the metal at more than one point, ensuring that the metal atom becomes part of a biologically stable ring structure. Many different assertions are made regarding the relative merits and suitability of amino acids versus peptides in forming mineral chelates with high bioavailability.
The role of bond strength on organic trace mineral stability
Most amino acids and peptides bind metal ions through either nitrogen, oxygen or sulphur atoms. Individual amino acids exhibit a range of stabilities when complexed with mineral which can be assessed in various databases.
Peptides, having a greater number of donor atoms which enable their potential to form numerous chelate rings when bonding to a metal ion, would be expected to form more stable chelates than simple amino acids, such as glycine. This, however, depends on the peptide’s ability to form more than one chelate ring.
Claims of superiority based on size have little merit in terms of stability. Simply increasing the number of amino acids in a ligand may not increase the stability of the metal complex nor the relative proportion of bound mineral. Ultimately, not only does the amino acid type influence stability but also the amino acids’ position in a peptide.
From a production standpoint, the extent and type of hydrolysis of a protein source to form short-chain peptides can significantly influence the sequence of amino acids present. Producing an ‘optimal’ protein hydrolysate for mineral chelation relies on carefully selecting the hydrolysis conditions, ensuring that the final hydrolysed peptide mix can guarantee constancy and mineral binding stability under conditions of changing pH.
Byrne et al. (2021) used potentiometric-based techniques to analyse a range of commercial OTMs. A Cu ion-selective electrode determined their in vitro stabilities over a pH range reflective of physiological conditions (Figure 1).
Figure 1- pH-dependant stabilities of copper chelates.
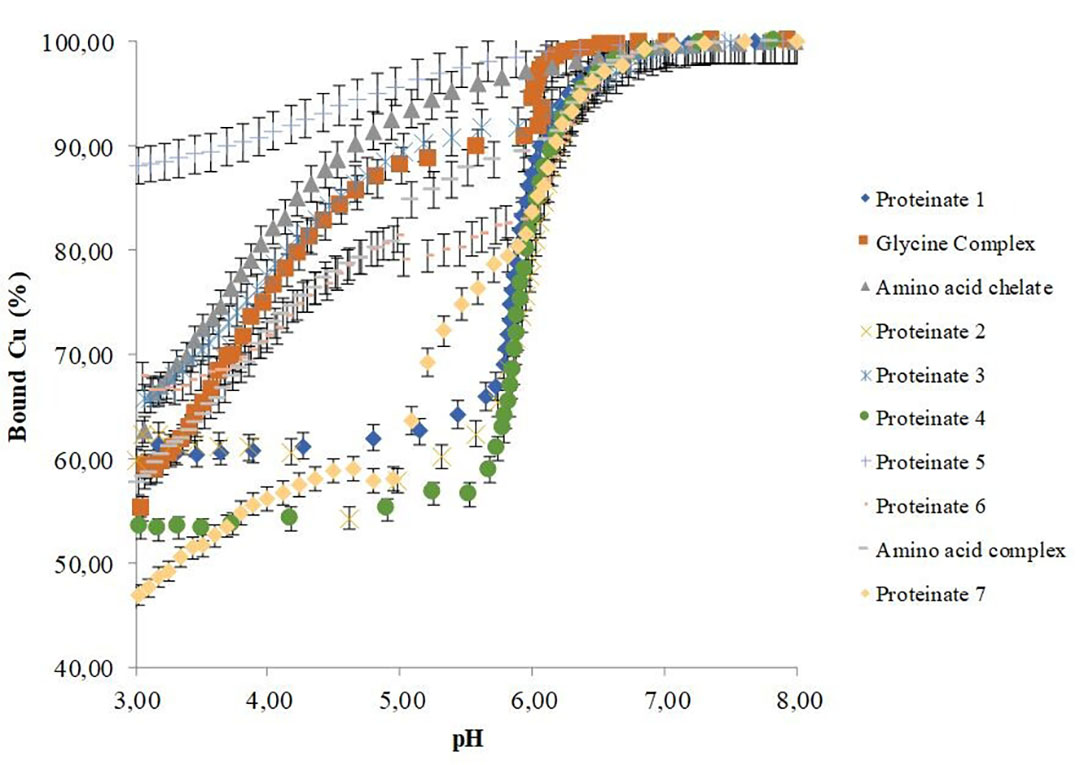
In this work, samples were reconstituted and suspended before titration of the supernatants, with subsequent measurement of the percentage bound copper over a pH range of 3–8. This confirmed that notable differences exist in the pH-dependant stability of commercial OTMs, with the amount of bound copper varying considerably between samples. Furthermore, the data indicates that some OTMs have low or no capacity for stable mineral bonding at acidic pH, with obvious impacts on the bio-efficacy of the products. These differences can be attributed to not only the type of bonding group used but also to the production process used to generate them.
Ultimately, the stability of an OTM is vital to its bioavailability. During transit through the GI tract and as the pH decreases or acidifies, all OTMs are subjected to physiological forces, which can result in the bound mineral complex dissociating and releasing free mineral ions.
This means that complexes or chelates with low stabilities will potentially have reduced bioavailability and effectiveness of the product to that of the corresponding inorganic salt. Maximising the pH-dependant stability of OTMs will increase mineral bioavailability and uptake in the intestine.
Influence of OTM stability on relative bioavailability
The absorption and utilisation of OTMs by an animal also differ greatly, and much research has been devoted to understanding the relative bioavailability of organic minerals, usually by comparison with inorganic mineral sulphates using a range of assessment parameters.
Bioavailability has many definitions, but in terms of trace minerals, it refers to the relative proportion of ingested mineral absorbed and retained by the animal species under study.
The white paper review highlights some examples of zinc source bioavailability studies in poultry. In general, organic zinc sources have greater bioavailability than their inorganic counterparts. Additionally, inter-study variability can be noted, which not only depends on the measurement index used to assess bioavailability but also the source used. In essence, differences in bioavailability can be observed even within groups of OTM).
Influence of OTM stability on relative bioavailability
The absorption and utilisation of OTMs by an animal also differ greatly, and much research has been devoted to understanding the relative bioavailability of organic minerals, usually by comparison with inorganic mineral sulphates using a range of assessment parameters.
Bioavailability has many definitions, but in terms of trace minerals, it refers to the relative proportion of ingested mineral absorbed and retained by the animal species under study.
The white paper review highlights some examples of zinc source bioavailability studies in poultry. In general, organic zinc sources have greater bioavailability than their inorganic counterparts. Additionally, inter-study variability can be noted, which not only depends on the measurement index used to assess bioavailability but also the source used. In essence, differences in bioavailability can be observed even within groups of OTM).
Many factors affect bioavailability, including animal species, sex, physiological state, existing mineral status, choice of response criteria, choice of standard source and chemical form and mineral element solubility. Concerning chemical form of the mineral, the chelation strength between the mineral and bonding group will define OTM stability and ultimately play a significant role in influencing relative bioavailability. A limited number of studies compare OTM chelation strength with bioavailability, demonstrating a direct link between OTM stability and bioavailability.
Key findings from several of these studies, show that as chelation strength increases, so too does the relative bioavailability of mineral source. Increasing the bond strength between the mineral and the bonding group used will, therefore, prove a very effective strategy to enhance OTM bioavailability.
Premix and feed antagonisms
Increasingly, the agonistic and antagonistic effects of feed components have come under scrutiny, with choice of components gaining increasing importance in diet formulation. The possibility for negative interactions between individual components within premixes and feeds is high and often overlooked, as are the underlying effects at a cellular level following digestion and absorption of the mineral source.
Recent studies have focused on assessing these potential antagonisms. The differential effects noted indicate that not all chelates are created equal; moreover, they differ in terms of stability, releasing mineral in a pH-dependent fashion based on the pH in the local micro-environment. This instability results in some chelates negatively impacting premix and feed components.
Conclusions
Despite confusing and often contradictory information, mineral chelation is a relatively straightforward process governed by some fundamental chemistry basics. By carefully considering factors important in mineral chelation, one can begin to distinguish between the products by their biological stabilities and bioavailability.
Ultimately, the stability of an OTM is essential to its bioavailability. During transit through the GI tract and as the pH decreases or acidifies, all OTMs are subjected to physiological forces that can result in the bound mineral complex dissociating and releasing free mineral ions. Organic trace minerals with optimised stability and bond strength will have far less potential for reactivity compared to inorganic sources. However, different forms of organic trace mineral will react differently and cause greater or less inhibition of feed components depending on source.
Lastly, diet formulators may well need to pay greater attention to their choice of individual components to minimise the financial costs associated with negative interactions, which could be significant.
For more information, click here to download the white paper, “Organic Trace Minerals: Enhancing mineral bioavailability through chelation” by Dr. Richard Murphy, director of research at Alltech.






