New rapid test developed for toxic ergot alkaloids

With contribution from a consortium of companies led by WFSR (Wageningen Food Safety Research, The Netherlands), Randox Food Diagnostics has developed an ELISA that is sensitive enough to measure ergot alkaloids around the limit value. The ELISA has been validated for rye, barley, wheat, oats and spelt, and no false negatives were found for the field samples tested.
Grain processors regularly face the presence of toxic ergot alkaloids (EAs). Europe has set new limits of detection since early 2022, but analysis is expensive and time-consuming. The industry therefore needs a faster, cheaper method to allow infected batches to be identified earlier. Thanks to a successful Top Consortium for Knowledge and Innovation (TKI, The Netherlands) project, led by WFSR, this method is now available.
Legislation
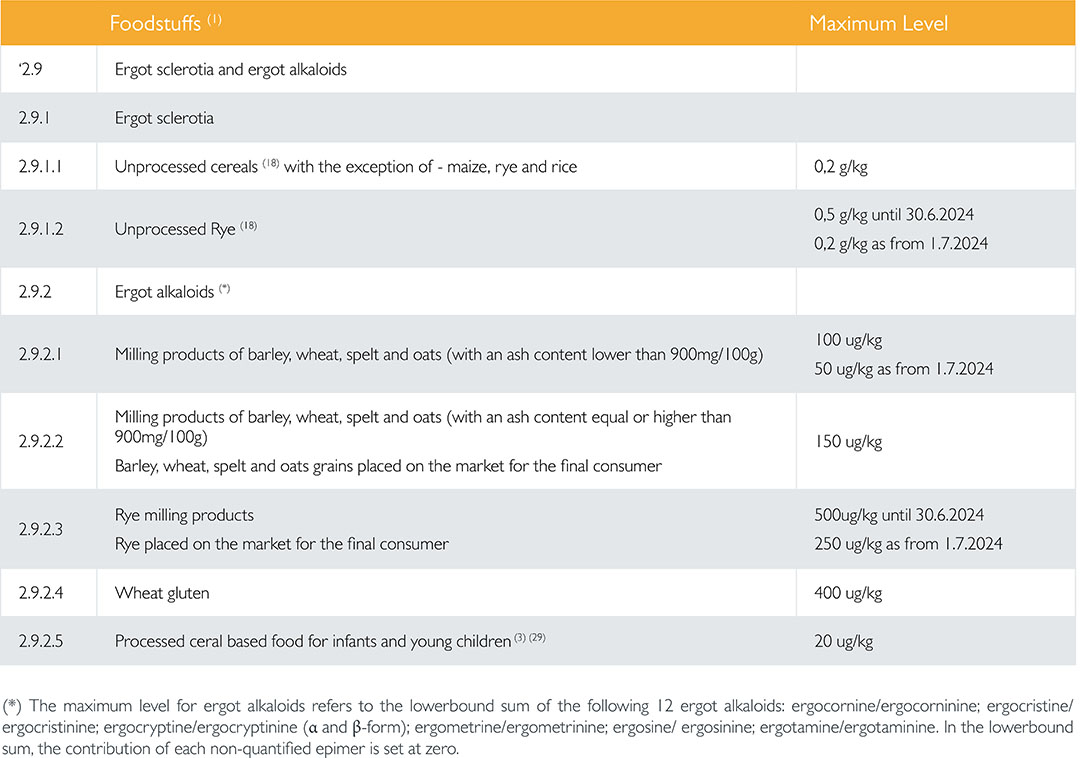
Since 1st January 2022, new standards have been established in European Regulation EU 2021/1399 for both the presence of sclerotia in cereals and toxins in the cereals or products derived from them. Table 1 gives an overview of the limits, detailing the 12 EAs which are regulated. EU 2021/1399 distinguishes between the occurrence of sclerotia and EAs, but also between the different grains and even the different products derived from cereals.
Furthermore, the European Commission (EC) expects that the EA content in flour will continue to fall due to process improvements. The EC is already anticipating on this by introducing lower standards in 2024, but it is not inconceivable that the EC will lower the limits even further in the future. The lowest limit applies to infant nutrition, with children and pregnant women appearing to be the most sensitive, and children having on average a higher cereal consumption per kg of body weight.
Regulation (EU) 2021/1399 has consequences for the monitoring of EAs and the management of contaminated batches of grain by companies. An example: a producer of infant nutrition should not use a batch of wheat with an EA content of more than 20 µg/kg for Brinta whole grain breakfast (limit 20 µg/kg), but perhaps can still use it for self-raising flour (limit 100 µg/kg).
Table 1
New method for ergot alkaloids
It is crucial for companies to monitor the level of EAs. One of the ways to prevent high levels of EAs from ending up in the end product, is to use a rapid test at the place where grains are stored or processed or a quick check of ready-to-eat products. Until now, EAs could only be measured with LC-MS/MS or HPLC-FLD. These methods are precise but also time-consuming and expensive.
In 2019, TKI Agri&Food financed a project (LWV19252) that aimed to contribute to the development of a validated ELISA test for EA in collaboration with industry partners. Access to practical samples, especially blank samples with the lowest possible level of EAs, was essential for the creation of a validated ELISA. The Committee of Grain Traders supplied the various grain samples used by WFSR and Eurofins for making QC materials. Randox Food Diagnostics used the QC materials for the first validation of the ELISA they developed. In a 2nd round using field samples, the ELISA test was validated by Randox Food Diagnostics, WFSR and Nutrilab as an independent third party.
The collaboration resulted in a useful ELISA test for detecting Ergot Alkaloids in rye, barley, wheat, oats and spelt.
Research design
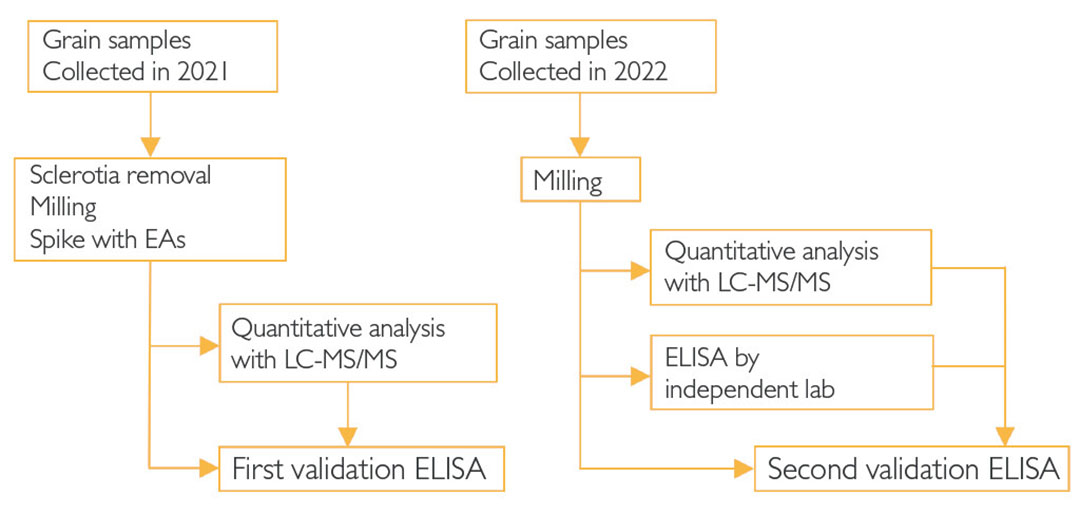
The project was divided into 2 phases. In phase 1 (2021, Figure 1), the grain traders supplied a total of 70 samples for 5 different grains. These samples were sanitised by manually removing all sclerotia present. Then the cereals were ground into a powder. EAs were added up to the maximum permitted limit concentrations according to EU 2021/1399. The EA levels were measured with a validated LC-MS/MS method and this data was used for the first validation of the newly developed ELISA. New grain samples were delivered in phase 2 (2022). These samples were ground without cleaning and divided into three portions. Aliquots were analysed independently for the presence of EAs. In this phase, the ELISA was performed by the kit developer (Randox Food Diagnostics) and by an independent laboratory (Nutrilab).
Figure 1
Results
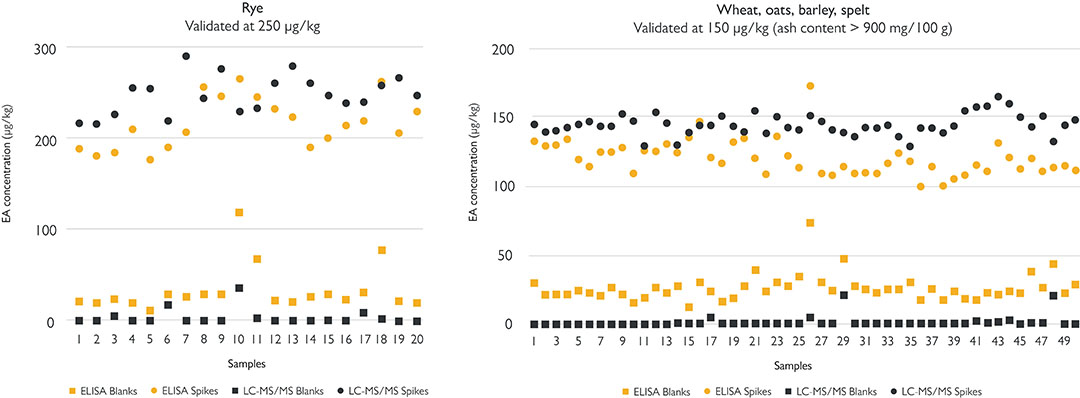
Figure 2 below shows results from phase 1. The squares represent the EA content of the cleaned blanks as measured by the LC-MS/MS method and the ELISA. The ELISA gives a slightly higher background than the LC-MS/MS. The dots represent the results of the grain samples spiked with the 12 different EAs in equal amounts to a total of 250 µg/kg for rye or 150 µg/kg for the other four grains. The LC-MS/MS and ELISA results are generally well matched. There are no false negatives, which means that the ELISA can play a valuable role in monitoring the food safety of cereals. The ELISA gives a level higher than 50 µg/kg for four blank samples, apparently a false positive result. But that has to be seen in context.
Figure 2
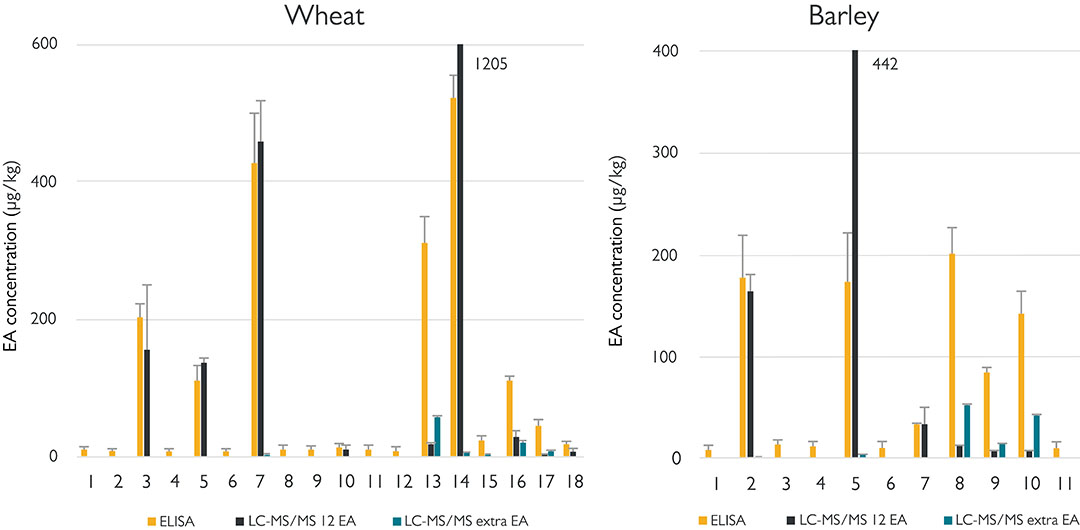
In phase 2, authentic samples were measured as seen in Figure 3. For all grains, some samples were found with an EA content higher than the legal limit, both by LC-MS/MS and the ELISA (see, for example, wheat samples 3, 7 and 14 in the figure below). This again illustrates the value of the ELISA, because on the basis of a positive result, the grain trader can, for example, decide to choose a different application for the batch of grain. Surprisingly, in some samples (wheat 13, 16) the ELISA gives a significantly higher level of EAs than the LC-MS/MS. In contrast to ELISA, LC-MS/MS sees all individual EAs: so in addition to the 12 regulated EAs, also EAs that may be present in the grain, but that are as yet unregulated. The ELISA cannot make that distinction and thus measures the sum of regulated and unregulated EAs. It is striking, and this can be clearly seen from samples 8, 9 and 10 of barley, that the ELISA seems to react more strongly to the presence of unregulated EA. Examples of this are the EAs of the clavine type. The ELISA can therefore more easily give a false positive result for grains containing unregulated EA. In the event of a positive ELISA, the grain trader can still consider having an additional chemical analysis carried out, which may then lead to the conclusion that the grain meets the legal requirements. In addition, it is important that the grain samples are ground properly and as finely as possible, so that a representative result is obtained. Sclerotia are difficult to grind and distribute homogeneously throughout the test sample.
Figure 3
Summary and Conclusions
- Since 1st January 2022 there are European limits in effect for the presence of EAs in cereal products.
- LC-MS/MS is the most common method for quantitatively measuring EAs. This method is precise, but also time-consuming and expensive. The industry needs a faster, cheaper method, so that contaminated batches can be identified earlier.
- With the contribution of a consortium of companies led by WFSR, Randox Food Diagnostics has developed an ELISA that is sensitive enough to measure EA around the limit value. The ELISA has been validated for rye, barley, wheat, oats and spelt, and no false negatives were found for the field samples tested.
- The ELISA requires only a simple sample preparation without the need for additional sample clean-up.
- The ELISA seems to be sensitive to some EA that are not regulated, such as the clavine type EA. These EA usually occur in lower concentrations than the twelve regulated ones but can nevertheless lead to false suspect samples.
Acknowledgement
The research was carried out in project LWV19252, Dutch initiative for evaluation and quality assurance of fast methods in food safety testing, which was funded by TKI Agri&Food. The partners involved were: The Committee of Grain Traders, Nutrilab BV, Eurofins Labco, Randox Food Diagnostics and Wageningen Food Safety Research. Read the full article here.
For more information on Randox Ergot Alkaloids ELISA, contact us here!


