Understanding the robustness and stability of the microbiome

The microbiome, often called the “forgotten organ,” plays a vital role in maintaining the health and well-being of animals. It consists of a diverse array of microorganisms, predominantly bacteria, that colonise the gut. A “robust” microbiome refers to a microbial community that is stable, resistant to disruption, and capable of quickly recovering from external stressors. A stable microbiome is essential to prevent dysbiosis, an imbalance that can lead to various health issues.
WORLD OF MICROBES SPECIAL 2024 – read all articles
A microbiome is more than just the microorganisms present in a specific environment; it encompasses their genetic material and the myriad biochemical processes they influence. It interacts with the host’s immune system, metabolism, and even the nervous system, particularly through the gut-brain axis. This interplay regulates crucial functions, from nutrient absorption to stress response.
The term “microbiota” refers specifically to the organisms themselves, while the “microbiome” includes the collective genomes of these microorganisms. For animals, a balanced microbiome is essential for optimal growth, productivity, and resistance to disease.
The evolutionary importance of microbiomes
Bacteria have existed on Earth for about 3.2 billion years, co-evolving with all higher life forms, including animals. This symbiotic relationship is foundational: all higher organisms have evolved in the presence of, and constant interaction with, bacteria. These microorganisms are essential for digestion, immune system function, and the maintenance of homeostasis in animals, with the gut microbiome playing a central role in these processes.
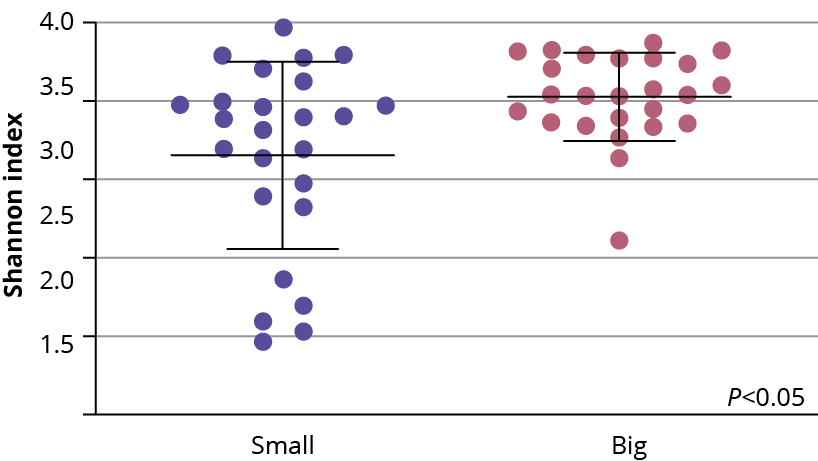
Figure 1 – Alpha diversity was higher in the microbiotas of the large birds compared to those of the small birds.
The science of a robust microbiome
The robustness of the microbiome is characterised by its alpha diversity and beta diversity. Alpha diversity refers to the variety of microorganisms within a single host, while beta diversity measures differences in microbial communities between different hosts. A higher alpha diversity is generally a sign of a healthy, stable microbiome, whereas a loss of diversity can indicate dysbiosis.
For instance, in broiler chickens, a study demonstrated that birds with higher microbial diversity exhibited greater resilience against stressors and performed better in growth metrics. The “big” birds in the study had significantly greater alpha diversity compared to their smaller counterparts (Figure 1). This higher diversity led to more uniform growth rates and lower susceptibility to illness. In the trial, birds with the most robust microbiomes had higher levels of short-chain fatty acid (SCFA)-producing bacteria, which positively influence gut health and the immune system.
The consequences of dysbiosis
Dysbiosis, or microbial imbalance, is linked to a variety of health problems in animals. In broiler chickens, for example, dysbiosis has been associated with reduced growth performance, higher mortality rates, and increased susceptibility to diseases like necrotic enteritis. The presence of pro-inflammatory microorganisms and the reduction of beneficial microbes lead to systemic inflammation, which can compromise animal health.
Dysbiosis can also disrupt the gut-brain axis in animals, leading to behavioural changes. In chickens, stress-related behaviours such as reduced feed intake and increased anxiety have been linked to gut microbiome imbalances. A robust microbiome, by contrast, promotes positive interactions through the gut-brain axis, reducing stress-related behaviours and improving overall animal welfare.
The role of a stable microbiome
A stable microbiome contributes to improved animal health by enhancing immune function and nutrient absorption. Beneficial microorganisms in the gut, such as Faecalibacterium and Butyricicoccus, are known to produce SCFAs, which strengthen gut barrier integrity and reduce inflammation. In a study on broiler chickens, birds with a higher abundance of SCFA-producing bacteria showed improved feed conversion ratios and body weight gains compared to birds with less diverse microbiomes.
This microbial stability is critical not only for performance metrics but also for disease prevention. By maintaining a diverse microbiome, animals are less likely to suffer from pathogen overgrowth, which can lead to gastrointestinal diseases and increased mortality. For example, poultry fed with probiotics showed an increase in the gut’s Bifidobacterium levels, improving resilience to gut infections and supporting healthier growth.
Practical applications: Enhancing microbiome health in animals
Promoting microbiome robustness through dietary interventions, such as the use of probiotics, has yielded significant benefits in animal production. In poultry, diets supplemented with probiotics resulted in increased alpha diversity of the gut microbiome, which translated into better growth rates and lower stress levels. In a study, broiler chickens fed probiotics had an average body weight of 7.07 lb at day 42, compared to 6.80 lb for the control group. Moreover, birds fed probiotics exhibited higher serotonin levels and lower corticosterone levels, leading to reduced stress behaviours such as pacing and feather pecking (Table 1).
Novonesis is an expert in biosolutions that support microbiome robustness and animal health. By developing tailored solutions that promote microbial diversity, Novonesis helps ensure the health and productivity of livestock, demonstrating the potential to address challenges in modern animal agriculture.
The future of microbiome research
Understanding the microbiome’s role in animal health is an evolving field with immense potential.
As science uncovers the ways in which the microbiome influences health and well-being, maintaining a robust and stable microbiome will remain central to ensuring the health of livestock. Promoting microbial diversity through biosolutions, such as those offered by Novonesis, will be key to enhancing productivity and animal welfare in agriculture.
References available on request.